- Submissions

Full Text
Associative Journal of Health Sciences
Effects of Narcotic Analgesics and M-Cholinoceptor Blocking Drugs on Sphincter Of Oddi Wave Propagations
Zhen-Hai Zhang*1, Zhong-Hou Rong1, Xin-Xing Wang1, Dong-Dong Du1, Jing Kong2s and Yang Su2
1 Department of Hepatobiliary Surgery, Shandong Provincial Hospital, China
2 Department of Minimally Invasive Surgery and the Second General Surgery, Shengjing Hospital, China
*Corresponding author: Zhen-Hai Zhang, Department of Hepatobiliary Surgery, Provincial Hospital Affiliated to Shandong University, No. 324 Jingwu Road, Huaiyin district, Jinan, Shandong Province, China.
Submission: July 29, 2020;Published: December 08, 2020

ISSN:2690-9707 Volume1 Issue3
Abstract
Background: Sphincter of Oddi (SO) manometry (SOM) was considered as the gold standard for evaluating SO motility [1]. It is hard to endure pain for patients just given diazepam sedation instead of analgesics during SOM. However, there are controversial for analgesics to alleviate biliary pain. Therefore, it is necessary to further study the mechanism of analgesics on SO. Previous studies [2,3] have used some regular indexes during SOM to assess effects of analgesics on SO. However, none of these indexes is specific or permanent. Among these indexes, SO wave propagation direction can also be important. However, it has rarely been calculated.
Aim: To investigate the effects of analgesics and M-cholinoceptor blocking drugs on SO wave propagation direction.
Methods: Patients with placement of T-tube after cholecystectomy and choledochotomy from 2011 to 2015 were retrospectively reviewed. They were divided into the morphine, Ap-237, pethidine, tramadol and control group. The morphine group was divided into three subgroups on the basis of the effects of M-cholinoceptor blocking drugs against morphine: buscopan, atropine and anisodamine group. The following data were collected for all patients: demographic details, time of T-tube drainage, SO wave propagation data and use of corresponding analgesic drugs. Percentages of antegrade, retrograde and indeterminate waves were scored and analyzed by means of choledochoscopic manometry.
Results: A total of 140 patients were included. Median age was 55.5 years, 45% were male. All patients underwent cholecystectomy and choledochotomy, at least 1.5 months (mean 2.5 months) after T-tube drainage. The percentage of antegrade waves decreased at 10(10.97±1.62 vs 74.09±8.55, 14.17±2.80 vs 74.09±8.55, P<0.001) and 20min(16.25±1.17 vs 74.09±8.55, 22.90±1.40 vs 74.09±8.55,P<0.001) after injection of morphine and Ap-237, respectively. The percentage of indeterminate waves increased at 10(72.91±12.60 vs 19.11±2.25, 58.33±3.63 vs 19.11±2.25, P<0.001) and 20min (64.82±9.66 vs 19.11±2.25, 42.76±3.47 vs 19.11±2.25, P<0.001) after injection of morphine and Ap-237, respectively. The percentage of retrograde waves also increased markedly at 10(27.50±3.15 vs 7.94±1.19, P<0.01) and 20 min (30.05±2.42 vs 7.94±1.19, P<0.001) after injection of Ap-237. Buscopan and anisodamine blocked wave propagation abnormalities induced by morphine. The percentage of antegrade waves decreased and percentage of indeterminate waves increased 10min after injection of tramadol, but 20min later, these changes were not significant. Changes in percentages of antegrade, indeterminate and retrograde waves were not significant 10min after injection of pethidine.
Conclusion: SO, wave propagation with choledochoscopic manometry is an accurate method to investigate SO motility. Pethidine and tramadol rather than morphine and Ap-237 are better choices to alleviate biliary pain.
Core tip: This study investigated the effects of analgesics and M-cholinoceptor blocking drugs against morphine on SO wave propagation direction. We found that wave propagation is disturbed after injection of morphine and Ap-237. Buscopan and anisodamine can block wave propagation abnormalities induced by morphine. Pethidine and tramadol rather than morphine and Ap-237 appear to be the choice to alleviate biliary pain. In addition, as an important index during SOM, percentage of SO wave propagation can be more accurate than other indexes to investigate SO motility.
Keywords: Narcotic analgesics; Sphincter of Oddi; Wave propagation; Manometry; Choledochoscopy
Introduction
SO is a smooth muscle sphincter located at the junction of the common bile duct and main pancreatic duct with the duodenum [4]. SO may control the emission of bile and pancreatic juice into the duodenum and prevent reflux of duodenal juice into the biliary and pancreatic ducts. SO, dysfunction (SOD) is a motility disorder. If SOD occurs, patients experience recurrent biliary-type pain in the abdominal area [5] and have a risk of recurrent pancreatitis [6]. SOM was considered as the gold standard for evaluating SO motility [1].
Morphine, pethidine, tramadol and Ap-237 are the most commonly used analgesics. Morphine can increase intra-biliary duct pressure, and delay bile flow into the duodenum, and induce upper abdominal pain with characteristics of biliary colic in some patients [7,8].
However, these results have been disputed by Thompson [9]. Furthermore, whether pethidine is the analgesic of choice in patients experiencing biliary pain is also controversial [8,10]. Due to potential harm, all narcotic analgesics, including pethidine, are prohibited for alleviation of biliary pain in patients with biliary and pancreatic diseases [9] or in patients examined with SOM. However, it is hard to endure pain for patients just given diazepam sedation instead of analgesics during SOM. Therefore, it is necessary to further study the mechanism of analgesics on SO.
Previous studies by the current authors Kong Jing & Zhang ZH [2,3] found that morphine and Ap-237 increased basal pressure of the SO, frequency and amplitude of phasic contractions during SOM. Morphine increased common bile duct pressure, tramadol decreased SO basal pressure and amplitude of phasic contractions, and pethidine had no effects on SO motility. However, none of these indexes is specific or permanent. Among these indexes, SO wave propagation direction can also be important. However, it has rarely been calculated. The aims of this study were:
A. To investigate the effects of analgesics and M-cholinoceptor blocking drugs on SO wave propagation direction.
B. To determine which analgesic is the better choice for relieving biliary pain.
C. To identify the importance of SO wave propagation duing SOM for evaluating SO motility.
Materials and Methods
Patients and drugs
Patients with placement of T-tube after cholecystectomy and choledochotomy at Shengjing Hospital of China Medical University between November 2011 and December 2015 were retrospectively analyzed with regard to the effects of analgesics and M-cholinoceptor blocking drugs on SO wave propagation direction. They were divided into the morphine group (n=40), Ap- 237 group(n=10), pethidine group(n=10), tramadol group(n=10) and control group(n=70). The morphine group was divided into three subgroups on the basis of the effects of M-cholinoceptor blocking drugs against morphine: buscopan group(n=10), atropine group(n=10) and anisodamine group(n=10).
The following data were collected by looking up the medical record and telephone interview for all patients: demographic details, time of T-tube drainage after cholecystectomy and choledochotomy, SO wave propagation data(percentages of antegrade, retrograde and indeterminate waves) acquired by means of choledochoscopic manometry and use of corresponding analgesic drugs. Patients who had undergone cholecystectomy and choledochotomy combined with partial hepatectomy or only cholecystectomy and patients with choledocholithiasis who had been subjected to a sphincterotomy or a balloon-sphincter-shaping procedure were excluded from the analysis.
Patients were given one of the four different analgesic drugs. All drugs were given intramuscularly. Morphine was injected at a dose of 0.1mg/kg after the first measurement. The second and third manometries were performed after 10 and 20min, respectively. 15mg anisodamine, 0.75mg atropine and 20mg buscopan were given 10min after injection of morphine in the morphine subgroups. Ap-237, pethidine and tramadol were injected at a dose of 1mg/kg. The procedures were same as the morphine group.
Sphincter of oddi manometry
Details of manometry have been described previously [2,3]. A triple-lumen polyethylene manometry catheter was used. The three side holes in the distal end were located 2mm apart. The catheter was introduced via the side pore of the choledochoscope directly into the duodenum. When the pressure was stable, the duodenal pressure curve was recorded. The catheter was then withdrawn in a stepwise fashion, and the position of the catheter in the SO was confirmed by direct observation through the choledochoscope. The percentages of antegrade, indeterminate and retrograde waves of SO were calculated and analyzed with PC polygraph HR (CTD-Synetics Medical Company, Swedish) and a special computer program.
Statistical analysis
Statistical analysis was performed using Student’s t test. Data were analyzed with SPSS version19.0 (SPSS Inc. Chicago, IL, USA), and P < 0.05 was set as the level of significance. The results were expressed as mean ± SE.
Results
Patient characteristics
A total of 140 patients with placement of T-tube after cholecystectomy and choledochotomy were retrospectively analyzed with regard to the effects of analgesics and M-cholinoceptor blocking drugs on SO wave propagation direction during the abovementioned time period. Median age was 55.5 years, 45% were male. There were 40 patients in the morphine group, 10 patients respectively in Ap-237, pethidine and tramadol group. There were 70 patients in control group. The morphine group was divided into three subgroups: buscopan, atropine and anisodamine group, and there were 10 patients respectively in three subgroups. All patients underwent cholecystectomy and choledochotomy, at least 1.5 months (mean 2.5 months) after T-tube drainage.
Effects of morphine on SO wave propagation
The percentage of antegrade waves decreased and the percentage of indeterminate waves increased at 10 and 20min after injection of morphine, respectively (P<0.001) (Table 1 & Figure 1).
Effects of Ap-237, tramadol and pethidine on SO wave propagation
The percentage of antegrade waves decreased and the percentage of indeterminate waves and retrograde waves increased markedly 10 and 20min after injection of Ap-237, respectively (P<0.001) (Table 1). The percentage of antegrade waves decreased and the percentage of indeterminate waves increased 10min after injection of tramadol (P<0.01), but 20 min later these changes were not significant compared to the control group. Changes in percentage of antegrade, indeterminate and retrograde waves were not significant 10min after injection of pethidine, but 20 min later the percentage of antegrade waves decreased and the percentage of retrograde waves increased (P<0.05).
Table 1: Effects of narcotic analgesic drugs on SO wave propagation.
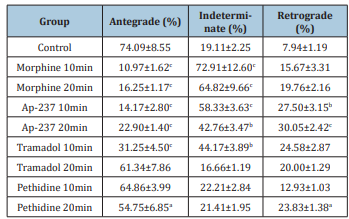
Significant differences between SO wave propagation: aP<0.05, bP<0.01, cP<0.001 vs control
Effect of M-cholinoceptor blocking drugs against morphine on SO wave propagation
Ten minutes after buscopan and anisodamine administration, abnormalities of wave propagation induced by morphine returned to normal. However, the percentage of antegrade waves did not increase obviously, and the percentage of indeterminate waves decreased, but not obviously, after injection of atropine (Table 2).
Table 2: Effects of M-cholinoceptor blocking drugs against morphine on SO wave propagation.
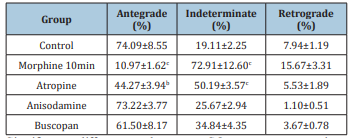
Significant differences between SO wave propagation: bP<0.01, cP<0.001 vs control.
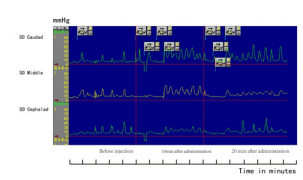
Figure 1: Manometric recording from SO illustrating changes in SO wave propagation at 10 and 20min after injection of morphine.
Discussion
SOM can be performed intraoperatively, or indirectly at the time of endoscopic retrograde cholangiopancreatography (ERCP) or performed via a T-tube by choledochoscopy. The application of SOM during ERCP is limited due to the complication of acute pancreatitis [11], the incidence of which can increase to 3.5% [12]. Performing SOM via a T-tube by choledochoscopy is an easy and accurate method of recording SO pressure [2,3]. The position of the manometric catheter in the SO can be monitored by direct observation through choledochoscopy. It is important to know into which system (common bile duct or pancreatic duct) the catheter is introduced, as measurement differences may exist.
SOM has been applied in many diseases including gallstones [13], common bile duct stones [14], idiopathic recurrent pancreatitis [15], and post-cholecystectomy syndrome [16]. Regular indexes measured during SOM include SO basal pressure, frequency and amplitude of SO phasic contractions, common bile duct pressure, and intrapancreatic ductal pressure. However, only a few but not all abnormalities in these indexes have been observed for some of the above diseases. In addition, none of these indexes is specific or permanent. For example, intrapancreatic ductal pressure was not significantly elevated in patients with acute recurrent pancreatitis compared with recurrent abdominal pain alone [17]. Among these indexes, SO wave propagation direction can also be important. However, as an important index, it has rarely been calculated to identify diseases with SOD. In this study, SO wave propagation direction was used as an important observation index measured during SOM. Percentages of antegrade, retrograde and indeterminate waves were scored and analyzed.
The normal percentages of wave propagations are disputable. One review report that antegrade and indeterminate waves account for 30-60% and retrograde 5-15% [18]. In a study of 26 patients, Abell et al. [19] found that wave propagations were 50% antegrade, 23% retrograde, and 27% indeterminate. The review of Toouli [20] showed that wave propagations were 79% antegrade, 9% retrograde, and 12% indeterminate. Our study of 140 patients with T-tube placement after cholecystectomy and choledochotomy found that wave propagations were 74% antegrade, 7% retrograde, and 19% indeterminate, which was in accordance with the findings of Toouli [20]. Antegrade waves comprised the majority, while retrograde and indeterminate waves accounted for only about 26% in the normal state.
We found that indeterminate waves increased to about 72% and antegrade waves decreased to about 11% 10min after injection of morphine during SOM in patients undergoing cholecystectomy and choledochotomy. Furthermore, these abnormal changes in wave propagation did not recover even 20min later. These wave propagation abnormalities can impede emission of bile and pancreatic juice into the duodenum and give rise to ischemia due to spastic contractions arising from increasing indeterminate wave propagation. Morphine can cause upper abdominal biliary colic and should not be used in these patients with biliary and pancreatic diseases. Studies by Butler and Thune support our results. Butler [7] found morphine precipitated pain consistent with biliary colic. Thune [8] found that morphine increased the mean frequency of contractions, and pethidine inhibited the frequency of contractions. So, the results [8] illustrated the suitability of pethidine over morphine as the analgesic of choice in patients experiencing biliary pain.
The effects of Ap-237 on SO wave propagation direction were the same as those of morphine. Percentage of indeterminate waves increased markedly to about 58%, and retrograde waves to about 27%, and antegrade waves decreased to about 14% 10min after injection of Ap-237. Furthermore, percentage of retrograde waves increased to about 30% 20min later. Therefore, Ap-237 should not be recommended to alleviate biliary colic in patients with biliary and pancreatic diseases, for the same reason as for morphine. Tramadol and pethidine have the same analgesic effect as morphine. The effects of pethidine and tramadol on SO wave propagation were not so obvious as those of morphine and Ap-237. Percentage of indeterminate waves increased to less than 50% 10 and 20min after injection of pethidine and tramadol, and percentage of indeterminate waves only increased to 16% 20min after injection of tramadol. At the same time, the percentage of retrograde waves did not increase obviously after injection of pethidine and tramadol. Studies by Staritz et al. & Brandstatter et al. [21,22] support our results. They evaluated the effect of tramadol on SO and found that it had no apparent effect on SO motility. Moreover, Shuo-dong & Wu et al. [3] indicated that tramadol decreased SO basal pressure and amplitude of phasic contractions, showing an inhibitory effect on the SO. Studies by Thune [8] illustrated the suitability of pethidine over morphine as the analgesic of choice in patients experiencing biliary pain. Therefore, pethidine and tramadol can be used to alleviate pain in the cases mentioned above. However, it should be noted that pethidine metabolite, normeperidine, might invoke seizures at high doses in patients with renal failure, hepatic failure or central nervous system diseases [10]. In addition, tramadol has little effect on the respiratory and circulatory systems and can be used in old patients with respiratory system diseases [10].
Our study also found that wave propagation abnormalities induced by morphine could return to normal after treatment with buscopan and anisodamine, which showed that M-cholinoceptor blocking drugs, except atropine, can resist biliary-type abdominal pain induced by morphine. In conclusion, we found that not all narcotic analgesic drugs should be prohibited for relieving biliary colic in patients with biliary and pancreatic diseases. In fact, pethidine and tramadol appear to be the analgesic drugs of choice. In addition, as an important index during SOM, percentage of SO wave propagation can be more accurate than other indexes to investigate SO motility.
References
- Kakuyama S, Nobutani K, Masuda A, Shiomi H, Sanuki T, et al. (2013) Sphincter of Oddi manometry using guide-wire-type manometer is feasible for examination of sphincter of Oddi motility. J Gastroenterol 48(10): 1144-1150.
- Wu SD, Kong J, Wang W, Zhang Q, Jin JZ (2003) Effect of morphine and M-cholinoceptor blocking drugs on human sphincter of Oddi during choledochofiberscopy manometry. Hepatobiliary Pancreat Dis Int 2(1): 121-125.
- Wu SD, Zhang ZH, Jin JZ, Kong J, Wang W, et al. (2004) Effects of narcotic analgesic drugs on human Oddi’s sphincter motility. World J Gastroenterol 10(19): 2901-2904.
- Woods CM, Mawe GM, Toouli J, Saccone GT (2005) The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 17(1): 31-40.
- Toouli J, Roberts IC, Dent J, Lee J (1985) Manometric disorders in patients with suspected sphincter of Oddi dysfunction. Gastroenterology 88(5): 1243-1250.
- Toouli J, Roberts T, Dent J, Lee J (1985) Sphincter of Oddi motility disorders in patients with idiopathic recurrent pancreatitis. Br J Surg 72(11): 859-863.
- Butler KC, Selden B, Pollack CV (2001) Relief by naloxone of morphine-induced spasm of the sphincter of Oddi in a post-cholecystectomy patient. J Emerg Med 21(2): 129-131.
- Thune A, Baker RA, Saccone GT, Owen H, Toouli J (1990) Differing effects of pethidine and morphine on human sphincter of Oddi motility.Br J Surg 77(5): 992-995.
- Thompson DR (2001) Narcotic analgesic effects on the sphincter of Oddi: A review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol 96(4): 1266-1272.
- Radnay PA, Brodman E, Mankikar D, Duncalf D (1980) The effect of equi-analgesic doses of fentanyl, morphine, meperidine and pentazocine on common bile duct pressure. Anaesthesist 29(1): 26-29.
- Zhang H, Cho J, Buxbaum J (2018) Update on the prevention of Post-ERCP Pancreatitis. Curr Treat Options Gastroenterol 16(4): 428-440.
- Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, et al. (2007) Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 102(8): 1781-1788.
- Rong ZH, Chen HY, Wang XX, Wang ZY, Xian GZ, et al. (2016) Effects of sphincter of Oddi motility on the formation of cholesterol gallstones. World J Gastroenterol 22(24): 5540 -5547.
- Feng YD, Zhang J, Jiao CH, Zhu H, Cheng WF, et al. (2017) Manometric measurement of the sphincter of Oddi in patients with common bile duct stones: A consecutive study of the Han population of China. Gastroenterol Res Pract 2017: 1-6.
- Fischer M, Hassan A, Sipe BW, Fogel EL, McHenry L, et al. (2010) Endoscopic retrograde cholangiopancreatography and manometry findings in 1,241 idiopathic pancreatitis patients. Pancreatology 10(4): 444-452.
- Isherwood J, Oakland K, Khanna A (2020) A systematic review of the aetiology and management of post cholecystectomy syndrome. Surgeon 17(1): 33-42.
- Fazel A, Geenen JE, MoezArdalan K, Catalano MF (2005) Intrapancreatic ductal pressure in sphincter of oddi dysfunction. Pancreas 30(4): 359-362.
- Funch JP, Ebbehøj N (1996) Sphincter of Oddi motility. Scand J Gastroenterol Suppl 216: 46-51.
- Abell TL, Werkman RF, Familoni BO, Baggous W, Massie D (1998) Biliary, pancreatic, and sphincter of oddi electrical and mechanical signals recorded during ERCP. Dig Dis Sci 43(3): 540-546.
- Toouli J (2009) Sphincter of Oddi: Function, dysfunction, and its management. J Gastroenterol Hepatol 24(Suppl3): S57-62.
- Staritz M, Poralla T, Manns M, Meyer Zum KH (1986) Effect of modern analgesic drugs (tramadol, pentazocine and buprenorphine) on the bile duct sphincter in man. Gut 27(5): 567-569.
- Brandstätter G, Schinzel S, Wurzer H (1996) Influence of spasmolytic analgesics on motility of sphincter of Oddi. Dig Dis Sci 41(9): 1814-1818.
© 2020 Zhen-Hai Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






