- Submissions

Full Text
Associative Journal of Health Sciences
Frequency of Associated Congenital Heart Defects in Down Syndrome
Hyder SN1, Humayun L2 and Kazmi T3
1,3 Department of Cardiology, Pakistan
2 Department of Hematology and Pathology, Pakistan
*Corresponding author:Hyder SN, Department of Cardiology, Pakistan
Submission: January 28, 2019;Published: February 27, 2019

ISSN:2690-9707 Volume1 Issue1
Abstract
Background: Patients with Down syndrome are prone to have congenital heart defects. Study was done to find the frequency of congenital heart defects in children with Down’s syndrome in Children Hospital Lahore.
Materials and methods:The descriptive study had directed by the Department of Cardiology in The Children’s Hospital and the Institute of Child Health, Lahore, in year 2017. One hundred and seven phenotypically Down syndrome children coming to the cardiology department for echocardiography from birth to 13 years were included in this study. The 2-dimension echocardiography had been done after detailed history and physical examination.
Results and discussion: Congenital heart defects were found in 63 out of 107 patients (59%). Among the affected patients, 68(63.5%) were males and 39(36.5%) females with ratio of 1.7:1. Among the isolated single lesions ventricular septal defect found in 60.3% patent ductus arteriosus in 13.7%, complete atrioventricular defects in 8.6%, followed by ASD in 6.8%, Pulmonary atresia with VSD in 6.8%, Tetralogy of Fallot and TGA in 1.7% of patients. Among the mixed lesion (4.7%) VSD+ASD found in two patients while COA+PDA, Univentricular heart with TGA and PA+VSD+DORV found in each patient.
Conclusion: Heart defects are found in 59% children with Down syndrome. The commonest in a cyanotic lesion are ventricular septal defect, in cyanotic cardiac lesion pulmonary atresia and VSD. In case of mix lesion ventricular septal defect with atrial septal defect were found.
Keywords: Down syndrome; Congenital heart disease; Transposition of great arteries; Pulmonary atresia; Tetralogy of fallot’s; Ventricular septal defect
Abbreviations:VSD: Ventricular Septal Defect; PDA: Patent Ductus Arteriosus; CAVSD: Complete Atrioventricular Septal Defect; ASD: Atrial Septal Defect; PA+VSD: Pulmonary Atresia+Ventricular Septal Defects; TOF: Tetralogy of Fallot’s; TGA: Transposition of Great Arteries
Introduction
Down syndrome is one of the common genetic disorder caused by the addition of partial or extra copy of chromosome 21 [1-4]. It is the most common genetic cause of moderate mental retardation. In developed countries the incidence of down syndrome varies from 1 in 732 live birth [1,5]. Cardiac anomalies associated with down’s syndrome include mainly patent ductus arteriosus (PDA), atrioventricular septal defect (AVSD), ventricular septal defect (VSD), atrial septal defect (ASD), tetralogy Of Fallot’s (TOF), coarctation of aorta (CoA), transposition of great arteries (TGA), double outlet right ventricle (DORV), pulmonary atresia/stenosis and univentricular heart [6]. Congenital heart diseases are the main factor contributing in the course of Down syndrome. Four to ten percent of all congenital heart diseases have association with Down syndrome and 40%-60% patients of Down syndrome have cardiac defects [7]. Cardiac malformations associated with Down syndrome have different frequencies in different countries. In USA and Europe atrioventricular septal defect (AVSD) seem to be the most common congenital heart defect. Ventricular septal defect is most common in Asian Countries, and atrial septal defect is most common in Latin America and Korea [6,8,9]. This study was conducted to see the frequency of different types of congenital heart defects among children with Down syndrome in Children Hospital Lahore with confirmation of karyotyping.
Materials and Method
A hospital-based descriptive study was conducted in the Department of Cardiology, Children hospital and Institute of Child Health Lahore. 107 males and females of Down syndrome aged from birth to 13 years attending Department of Cardiology in the Children’s Hospital Lahore for echocardiography diagnosed on phenotypic appearance were consecutively selected, over a period of year, from January to December 2017, irrespective of presence or absence of any symptom, sign, x-ray chest or ECG abnormality to suggest congenital heart defect (CHD). After detailed history and at least ten phenotyping findings, these children were subjected to 2-dimensional echocardiography in addition to routine chest x-ray, ECG and without karyotyping. Phenotypic findings included like mongoloid faces, brachycephaly, depressed nasal bridge, protruding tongue, small low set ears, upward slanted eyes with epicanthic fold, short neck, short and broad hands, transverse single palmar crease, clinodactyly, a large space between toes (sandal gap), hypotonia and developmentally delayed.
Exclusion criteria was:
a) PFO
b) PDA with premature baby below 4 weeks of life
c) And multiple CHD placed wide with dominant defect Data was analyses by SSPS 22 and frequency was applied.
Results
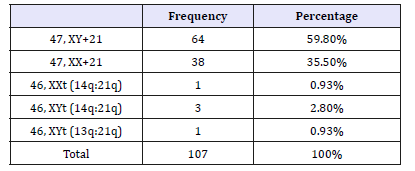
In 107 patients of Down syndrome, 63.5% (n=68) were males and 36.5% (n=39) females with male to female ratio of 1.7:1 and of these patients in which congenital heart defects were diagnosed were 59% (n=63). Patients were categorized in four age groups. Group-1 included below 2 months, group-2 included from two months to 1 years, group-3 included from one to five years and group-4 more than five years of age. Majority of patients belong to bellow five years of age (Table 1). Most of the congenital heart defects were found in bellow two months of age is 80%, between three months to one year 65% patients had congenital heart defects, bellow five years 57% patients had CHD while above five years 22% patients found to had CHDs. Congenital heart defects were categorized into cyanotic and a cyanotic, isolated and mixed lesions. Among the isolated lesions ventricular septal defect (VSD) was most common i.e. 60.3%, then PDA i.e. 13.7% and complete atrioventricular septal defect (CAVSD) (8.6%). In case of cyanotic cardiac lesion common lesion was Pulmonary atresia with VSD i.e. 5.17% then TOF 3.4% followed by TGA 1.7%. (Table 2)
Table 1:Age breakdown of Study population (n=107).
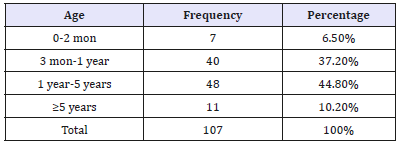
Table 2:Frequency distribution of isolated cardiac lesions with major categories of a cyanotic and cyanotic lesions among 107 cases.
PDA: Patent Ductus Arteriosus; CAVSD: Complete Atrioventricular Septal Defect; VSD: Ventricular Septal Defect; ASD: Atrial Septal Defect; Pulmonary Atresia+Ventricular Septal Defects; TOF: Tetralogy Of Fallot’s; TGA: Transposition of Great Arteries.
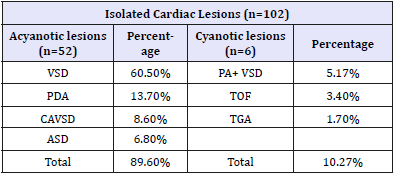
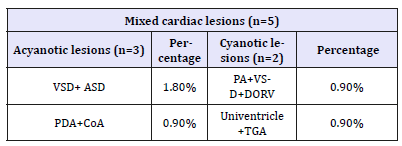
Among mixed lesions ventricular septal defect with atrial septal defect (VSD+ASD) found in 1.8%, Coarctation and PDA, double outlet right ventricle +pulmonary atresia (DORV+pulmonary atresia) and univentricle with TGA were found 0.9% each (Table 3). Regarding Karyotyping findings 47,XY+21 found in 64 patients i.e. 59.8%,38 patients were 47,XX+21,one patients with 46,XXt (14q:21q),three patients had 46,XYt (14q:21q),and one patients has 46,XYt (13q:21q) (Table 4).
Table 3:Frequency distribution of mixed cardiac lesions with major categories of a cyanotic and cyanotic lesions for 107 cases.
VSD+ASD: Ventricular Septal Defect and Atrial Septal Defect; PDA+COA: Patent Ductus Arteriosus; DORV+PA+VSD: Double Outlet Right Ventricle with Pulmonary Atresia and Ventricular Septal Defect; Univentricle+ TGA: Univentricular and Transposition of Great Vessels.
Table 4:Karyotypes (n=107).
Among mixed lesions ventricular septal defect with atrial septal defect (VSD+ASD) found in 1.8%, Coarctation and PDA, double outlet right ventricle +pulmonary atresia (DORV+pulmonary atresia) and univentricle with TGA were found 0.9% each (Table 3). Regarding Karyotyping findings 47,XY+21 found in 64 patients i.e. 59.8%,38 patients were 47,XX+21,one patients with 46,XXt (14q:21q),three patients had 46,XYt (14q:21q),and one patients has 46,XYt (13q:21q) (Table 4).
Discussion
The high incidence of congenital heart disease in Down’s syndrome is well known, and many authors have published figures on the frequency with which congenital heart defects are found. These figures vary from 35 to 65 per cent [1,10,11]. The frequency of CHD in this study 59% is quite comparable to these studies. It is quite close to 56.9% in Korea [6] and 56.36% in Khyber Pakhtunkhwa province in Pakistan [1]. The frequency of congenital heart defects is quite higher 81% in Brazil [12] and comparatively lower frequencies 45.10% in Libya [3] and 43% in Netherlands [13]. The various reasons for this difference may include the genetic make-up of each nation and the specific embryological mechanisms [1]. In 107 patients with Down’s syndrome, 68 (63.5%) were males and 39 (36.5%) females with male to female ratio of 1.7:1 and of these patients in which congenital heart defects were diagnosed were 63 (59%) are comparable to 61.3%. Male to female ratio of 1.7:1 as compare in Khyber Pakhtunkhwa province [1]. These findings are quite different in Brazil where females prevailed (56.1%) with male to female ratio of about 1:1.3. [12]. The most common type of congenital heart defects was isolated VSD in our study group i.e. 35 (60.5%) these values are very close to 90.3% in Peshawar [1] and are comparable to the Libyan population with 65% isolated lesion, 80% in Guatemala, [3,13] 74% in Mexico [3,7] Korea [6] and 78% in Turkey [3,14].
This difference may be because of age at diagnosis where patients with more complex lesions die earlier before diagnosis [1]. Multiple cardiac defects were present in 4.6% cases in our study. Among the a cyanotic isolated and mixed lesions most common defects were ventricular Septal defect (VSD), patent Ductus Arteriosus (PDA) and complete atrioventricular septal defect (CAVSD) followed by atrial septal defect with ventricular septal defect (ASD+VSD) in 2 (1.8%) and coarctation of aorta and patent ductus arteriosus (CoA+PDA) in 0.8%. Among the Cyanotic lesions isolated pulmonary atresia with VSD was more common in 5.17% followed by tetralogy of fallouts (TOF) i.e. 3.4% and transposition of great arteries (TGA) in 1.7% and mixed lesions of univenticle+ASD with atrial septal defect (univenticle+ASD) and double outlet right ventricle and pulmonary atresia (DORV +Pulmonary atresia) in 0.9 %. These findings were quite different from the findings in Korean children in which atrial Septal defect (ASD) was most common about 30.5% 2nd most common was ventricular Septal defect (VSD) with percentage of 19.3% this was followed by patent Ductus Arteriosus (PDA) (17.5%) and AVSD (9.4%) [6] and also in Brazil ASD second type was maximum (51.8%) followed by atrioventricular Septal defect (AVSD) (46.6%) ventricular Septal defect (VSD) 27.7% tetralogy of Fallot’s (TOF) (6.3%) and other cardiac anomalies (12.5%) [13]. In Sudan AVSD was most common in about (48%) followed by ASD in 23% and TOF in (6%) at the time of presentation (10%) had Eisenmenger syndrome [15]. The results were quite comparable to Indian population where atrioventricular septal defect (AVSD) was most common in about 13 (37.142%) among 35 down’s syndrome patients, while ventricular Septal (VSD) in 26 (68.421%) was most common among the 38 non-syndromic patients [16]. The striking feature of our study was the presence pulmonary atresia with ventricular septal defect as the most common cyanotic lesion that is 5.17% while in a cyanotic lesion VSD is found to commoner than ASD. Similarly, regarding karyotyping findings 95.3% patients had non-dysjunction,3.7% patients had translocation at 14q:21q and 0.93% had translocation at 13q:21q.
Limitation
The figures reported herein are not population based and of only one center. Also, the cytogenetic studies were not performed, and the diagnosis was mainly based on clinical grounds. As a result, we could not comment on the frequency of CHD in different chromosomal alterations of down syndrome.
Conclusion
Congenital heart defects are found in 63% children with Down syndrome. The commonest in a cyanotic cardiac lesion are ventricular septal defect, in cyanotic cardiac lesion pulmonary atresia and in mix type VSD and ASD were found in our center.
Ethics Committee Approval
Taking approval through ethical committee of The Children Hospital and Institute of Child Health, Lahore.
Consent form filled after taking consent from mother and father.
References
- Khan I, Muhammad T (2012) Frequency and pattern of congenital heart defects in children with down’s syndrome. Gomal J Med Sci 10(2): 241- 243.
- Kolgeci S, Kolgeci J, Azemi M, Beqiraj RS, Gashi Z, et al. (2013) Cytogenetic study in children with down syndrome among kosova albanian population between 2000 and 2010. Mater Sociomed 25(2): 131-135.
- Elmarpy Z, Rayani A, Shah A, Habas E, Aburawi E (2011) Down syndrome and congenital heart disease: why the regional difference as observed in the Libyan experience? Cardiovasc J Afr 22(6): 306-309.
- Aburawi EH (2006) The burden of congenital heart disease in Libya. Libyan J Med 1(2): 120-122.
- Sherman SL, Allen EG, Bean LH, Freeman SB (2007) Epidemiology of down syndrome. Ment Retard Dev Disabil Res Rev 13(3): 221-227.
- Kim MA, Lee YS, Yee NH, Choi JS, Choi JY, et al. (2014) Prevalence of congenital heart defects associated with down syndrome in korea. J Korean Med Sci 29(11): 1544-1549.
- Figueroa JR, Magaña BP, Hach JLP, Jiménez CC, Urbina RC (2003) Heart malformations in children with down syndrome. Rev Esp Cardiol 56(9): 894-899.
- Hamerton JL, Briggs SM, Giannelli F, Carter CO (1961) Chromosome studies in detection of parents with high risk of second child with down’s syndrome. Lancet 2(7206): 788-791.
- Castilla EE, Rittler M, Dutra MG, Camelo JSL, Campaña H, (1998) Survival of children with down syndrome in south America. ECLAMC-Downsurv group. Latin American collaborative study of congenital malformations. Am J Med Genet 79(2): 108-111.
- Bower C, Ramsay JM (1994) Congenital heart disease: a 10-year cohort. J Paediatr Child Health 30(5): 414-418.
- Abbag FI (2006) Congenital heart diseases and other major anomalies in patients with Down’s syndrome. Saudi Med J 27(2): 219-222.
- Mourato FA, Villachan LRR, Mattos SS (2014) Prevalence and profile of congenital heart disease and pulmonary hypertension in Down syndrome in a pediatric cardiology service. Rev Paul Pediatr 32(2): 159- 163.
- Vida VL, Barnoya J, Larrazabal LA, Gaitan G, Garcia FM, et al. (2005) Congenital cardiac disease in children with Down’s syndrome in Guatemala. Cardiol Young 15(3): 286-290.
- Nisli K, Oner N, Candan S, Kayserili H, Tansel T, et al. (2008). Congenital heart disease in children with down’s syndrome: Turkish experience of 13 years. Acta Cardiol 63(5): 585-589.
- Ali SK (2009) Cardiac abnormalities of Sudanese patients with down’s syndrome and their short-term outcome. Cardiovasc J Afr 20(2): 112- 115.
- Sharma BM, Khera MS, Sondhi LCV, Devgan CA (2013) A study to determine the prevalence of pulmonary arterial hypertension in children with down syndrome and congenital heart disease. Med J Armed Forces India 69(3): 241-245.
© 2019 Ekedahl RG. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






