- Submissions

Full Text
Associative Journal of Health Sciences
Quantitative EEG at Stimulation Correlated to Post-Stimulus Parameters in Electroconvulsive Therapy
Ekedahl RG*
Department of NeuFydi Stockholm, Sweden
*Corresponding author:Ekedahl RG, NeuFydi Stockholm, Sweden
Submission: February 06, 2019;Published: February 21, 2019

ISSN:2690-9707 Volume1 Issue1
Abstract
Introduction: The aim for present study is to evaluate epileptic activity induced by stimulation during electroconvulsive therapy (ECT) and to compare this activity with post-stimulus electroencephalographic (EEG)-parameters.
Materials and methods: A total of 135 EEG recordings of ECT sessions analyzed from 23 patients treated for depression. The recording site in the parietal cortex (P4), and stimulation performed at right unilateral or bi-temporal locations. The epileptic activity in sedated patients (i.e., activity in the 5-48Hz range of a total observed frequencies range of 0.5-48Hz), and the frequency distribution during stimulation and commonly used parameters in the post-stimulus phase evaluated. For statistical comparison of calculated parameters, the Pearson product-moment correlation coefficient used.
Results and discussion: The electrical shocks triggered EEG peaks in Delta to Gamma frequency bands and seemed dependent on stimulation frequency, with higher peak frequencies of responses for 70Hz than for 60 and 50Hz. A total of 15/23 patient had 90% or more of epileptic activity, while the remainder (8/23) had less than 90% epileptic activity in recorded sessions. Weak or no correlations observed when the proportion of epileptic activity and peak frequency during stimulation were compared with post-stimulus parameters. Even though no certain conclusions about the relationship of the antidepressant effect to the amount or the frequency of epileptic activity during triggered shocks from this study, a more effective stimulation been shown to give a better treatment effect of depressions.
Conclusion: EEG during stimulation may better evaluate the efficacy of stimulation than post-stimulus parameters and potentially improve ECT therapy by increasing the number of successful electroshock sessions in the treatment series.
Keywords: Electroconvulsive therapy; Electroencephalography; Epilepsy; Depressive disorder; Seizures; Electroshock; Brain; Humans; Anesthesia; Neurons
Abbreviations: EEG: Electroencephalography; ECT: Electroconvulsive Therapy; FFT: Fast Fourier Transform; AC: Alternating Current
Introduction
Since Ugo [1] introduced electroconvulsive therapy (ECT) for the treatment of patients with schizophrenia, this approach used for various psychiatric disorders, especially depression with psychotic features [1,2], several evaluations have demonstrated that ECT is an effective treatment for depression [3]. With the introduction of muscle relaxants and anesthesia to modern ECT techniques, monitoring the brain’s response has become more critical to control the efficacy of the applied electrical shocks. The commonly used anesthetics thiopental (Pentothal™) and propofol (Diprivan ™) exert antiepileptic effects and among other factors make individual recommendations for stimulus intensity difficult. These circumstances create a need to monitor electrical activity evoked in the brain by the electroshocks, and to identify EEG parameters related to satisfactory stimulation effect. Current computerized monitoring and automated analysis techniques which are incorpo rated in commonly used electrical stimulation devices such as the Thymatron™ (Somatics, LLC, Lake Bluff, Illinois, USA) and MECTA™ (MECTA Corp., Portland, Oregon, USA) these systems have limitations regarding accurate EEG evaluation throughout the ECT session. One significant limitation is that the recording starts after the electrical stimulation aborted, why these systems cannot evaluate epileptic activity during the actual electroshock (tonic phase). The recorded and analyzed post-stimulus EEG activity during an ECT session principally represents the clinic phase of an epileptic seizure (Figure 1) [4,5]. The post-stimulus parameters in automated EEG analysis (seizure duration, postictal suppression, and mid-ictal amplitude) have shown conflicting results how well they reflect antidepressant effects and the efficacy of stimulation during ECT [6-12]. By direct EEG recordings during the actual electrostimulation and compar- ison with the post-stimulus EEG parameters, can make it possible to evaluate which of these parameters that best reflect triggered activity by the electrical shocks. Additionally, recording and evaluation during electrostimulation may be a better method to predict the result of a successful ECT session for the individual patient. The proposed record and assessment of epileptic activity during the actual stimulation is also likely to improve stimulation techniques and result in better ECT treatment.
Materials and Method
Materials
A total of 135 ECT sessions were recorded and analyzed from 23 patients (7 men and 16 women, aged 24-82 years) recruited from a psychiatric clinic while undergoing ECT treatment for depression. As the objective of this study was not to evaluate ECT as such, but rather to determine how well post-stimulus parameters reflect the efficacy of triggered epileptic activity during stimulation, no further details concerning the patients are discussed. Patients were anesthetized with thiopental (Pentothal™) or propofol (Diprivan ™), ventilated, and administered a muscle relaxant before electric shocks delivered according to standard ECT unit procedures, including monitoring and good clinical practice. The local ethics committee of Karolinska Institute (Stockholm, Sweden) approved the analysis of the recordings.
Electrical stimulation
The electrical stimulation delivered to the right temporal or bi-temporal regions. Different stimulation intensity and pulse frequencies applied of 60 or 70Hz, in 3 of the sessions a 50Hz pulse frequency used. All electrostimulations performed with normal ECT unit routines. A standard Thymatron™ electroconvulsive device (Somatics, LLC, Lake Bluff, Illinois, USA) was used to trigger electric shocks for 8 seconds delivering square shocks with a duration of 0.25-1 millisecond (4000-1000Hz).
EEG recording
Standard subdermal needle electrodes used to provide stable impedance and recordings without disturbance of movement artifacts due to ventilation, stimulation or convulsions. Electrodes positioned at P3, P4 and Cz (vertex region) according to the International 10-20 System, with Cz, used as the reference. This positioning of electrodes minimizes possible muscle activity due to the absence of muscles in this area. Recordings were analyzed from P4 (right side) to ensure maximal responses for right unilateral temporal shocks. Contamination from stimulation artifacts avoided by the reference electrode (Cz) positioned between the stimulation and recording positions. Analyses were filtered to avoid supply line interference using a low-pass filter set at 48Hz (Figure 1 & 2). When the stimulation pulse frequency was analyzed, the high cutoff filter was set at 80Hz (Figure 1).
Figure 1:Electroencephalographic (EEG) recording of electroconvulsive therapy (ECT) session. Recording started (top left) when the patient anesthetized before electroshock (A), the electroshock in the middle top section with high amplitude (B) followed by the post-stimulus section top right (C). The three boxes indicated in the top recording show 8-second intervals from the different parts of the session (A,B and C) and the frequency analysis for each box shown in frequency diagram at the lower part of the illustration. Pre-and post-stimulus diagrams (A,C) dominated by the lowest frequencies (0.5- 5Hz), as expected in a anesthetized patient, and a 50Hz peak representing supply line interference..
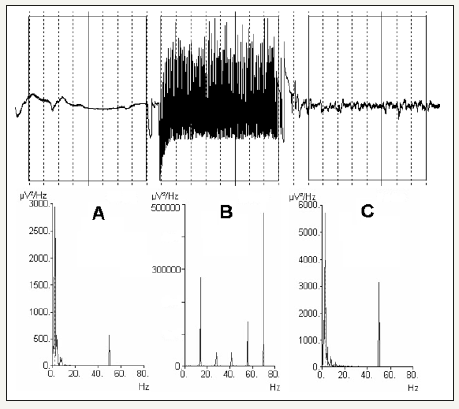
Figure 2:Different distributions of frequencies triggered by electroshocks. From the left, (A) a 2Hz peak was observed with 33% epileptic activity (5-48Hz) the stimulus pulse evoked frequency peaks dispersed around 60Hz. In (B) another response to 60Hz stimulus, dominant peaks at 28 and 33-34Hz and the stimulation pulse peak at 60Hz, 98% epileptic activity (5-48Hz). In (C) 70Hz stimulus pulse and a peak response at 70Hz and distribution of additional peak frequencies at 33-34Hz and 42Hz, 99% epileptic activity (5-48Hz). Triggered stimulation peaks had very high amplitudes, the maximum value of 500K μV2/Hz (see diagrams) representing hyper-synchronous evoked responses from neurons. The y-axis represents amplitude in μV2/Hz, and the X-axis represents frequency in Hz.

The recordings were continuously running and started when the patient was anesthetized, continued during and after stimulation until postictal suppression had passed (Figure 1). A Nervus™ 5.3 commercially-available digital EEG system (Viasys Healthcare, Inc., San Diego, California, USA) used for recording and analysis of EEG signals. The digital amplifier had a sampling frequency of 128Hz, and digital filters set to 0.5Hz low cutoff and a 48Hz high cutoff at analyses which permitted faithful reproduction of frequencies according to the Nyquist-Shannon sampling theorem [13].
Signals and statistical analysis
A fast Fourier transformation (FFT) used to digitize the analog EEG signal. The obtained frequencies divided into frequency bands of 0.5 to 5Hz and 5 to 48Hz. The 5 to 48Hz band should reflect epileptic activity for anesthetized patients during stimulation, 5Hz classified as the lowest frequency for epileptic discharges (sharp waves) [14,15], and the 0.5 to 5Hz band considered to indicate non-epileptic activity in anesthetized patients (Figure 1), [16]. The post-stimulus postictal suppression in the post-stimulus phase defined as the lowest identified amplitude, the ictal duration was measured from the time when stimulation aborted until postictal suppression appeared and the mid-ictal amplitude as the highest post-stimulus amplitude. The values of these post-stimulus parameters were calculated from digital data by an analyst trained in EEG analysis (the author) and used for further statistical comparison with the stimulus data in the analyses. The correlation between different parameters in the analysis was evaluated using Pearson product-moment correlation coefficients, where a value of 0 represents no correlation, 1 represents complete correlation, and -1 represents complete inverse correlation Figure 3.
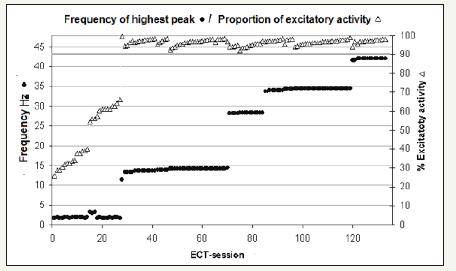
Figure 3:Electroconvulsive therapy (ECT) sessions sorted by epileptic activity. The dominant peak frequency of the electroshock response (primary Y-axis) and the percentage of excitatory epileptic activity (5-48Hz of a total frequency range of 0.5-48Hz) shown in secondary Y-axis. The frequency of the dominant peak is indicated by ● and the percentage of excitatory epileptic activity by Δ in the diagram. The X-axis represents ECT sessions. Note the horizontal shadowed line drawn from the 90% excitatory epileptic value on the secondary Y-axis.
Results and Discussion
Result
In all 135 analyzed sessions, patients reacted to electroshocks with a tonic response followed by clonic convulsions after stimulation aborted, indicating that the muscles were not completely relaxed. Therefore, it was not possible to evaluate the efficacy of stimulus from the visually observed seizures. Of the 138 originally recorded sessions, 3(2%) excluded due to supply line interference during stimulation. An additional 12 sessions (11% in total) the analyze of post-stimulus parameters were excluded due to movement artifacts in the post-stimulus phase. EEG analysis of the 135 electrical stimulation sequences revealed varying frequency patterns and distributions of a different type than in post-stimulus sequences (Figure 1 & 2). The frequency patterns divided into responses with different dominant peak frequencies (Figure 2). The frequency distribution in the post-stimulus phase had a similar frequency distribution that at stimulation, when a dominant peak-frequency of 2-3Hz and with the lowest percentage of 5-48Hz epileptic activity, indicating an inadequate response to that stimulus (Figure 1 & 4). Other sessions showed dominant peak frequencies at 11, 14, 28, 33-34 and 42Hz. When a 60Hz stimulation pulse frequency used, the dominant peak appeared at either 2 or 33-34Hz, the 2Hz dominant peak response sometimes also showed a second peak with a lower amplitude at 10-11Hz. When the dominant peak was 33-34 Hz, second peaks at 9Hz and 25Hz with lower amplitudes also appeared (Figure 2). The 70Hz stimulation pulse frequency revealed dominant peak frequencies at 14, 28 or 42Hz, peaks with varying lower amplitudes of 13-14Hz, 27-28Hz and 42Hz also present in addition to the dominant peak (Figure 2). When 50Hz stimulation used, dispersed peak at approximately 3Hz appeared without additional peaks.
A total of 27/135 sessions (20%) exhibited a dominant peak at 2-3Hz and less than 90% of 5-48Hz epileptic activity, while (80%) had dominant peaks at either 11, 14, 28, 34 or 42Hz and 90% or more of 5-48Hz epileptic activity (Figure 3). The correlations between the proportion of 5-48Hz epileptic activity during stimulation and the postictal maximum amplitude were weak (0.3), low for postictal suppression (-0.1), and absent for postictal duration (0.0). The dominant peak frequency in the responses also had low correlations with postictal maximum amplitude (0.2), postictal suppression (0.0) and postictal duration (0.1). Additionally, the correlation coefficients were low (ranged from -0.1 to 0.1) when the post-stimulus parameters compared to each other. The correlation between the percentage of 5-48Hz epileptic activity and dominant peak frequency during stimulation was relatively good, with a correlation coefficient of 0.7 Comparisons of age and all other parameters yielded 0.0 correlation coefficients. Analysis of 5-48Hz, epileptic activity in ECT sessions during stimulation, demonstrated that 15/23 patients had epileptic activity greater than 90% in all recorded sessions, while the remainder (8/23) patients had some sessions with lesser than 90% epileptic activity of recorded ECT sessions. (Figure 4). This circumstance may indicate a poorer outcome of stimulation during ECT treatment series for the latter group of patients. However, the results of treatment cannot be evaluated by this study because varying numbers of ECT sessions for individual patients (2-9 sessions) and no psychiatric evaluation performed.
Figure 4:Electroconvulsive therapy (ECT) sessions for individual patients. Sorted of the percentage of 5-48Hz excitatory epileptic of 0.5-48Hz and by year of birth for the patient, which identifies individual patients, shown at Y-axis. The horizontal rows indicated by filled circles (●) represent different sessions for the same patient (X-axis) and triangles (Δ) percentage excitatory epileptic activity (Y-axis in the top diagram). For several individuals, some electroconvulsive therapy (ECT) sessions in the treatment series had less than 90% excitatory epileptic activity, while others showed more than 90% activity in all sessions, illustrated by two different patients ECT-sessions shown in separated magnified diagrams in the lower segment of the figure. The left lower diagram from a patient born in 1967 whose first two sessions exhibited more than 90% activity, while subsequent sessions clearly show less than 90% excitatory activity and right lower diagram from a patient born in 1984 and with more than 90% activity in all nine sessions.

Discussion
Technical: The obvious technical difficulty of EEG recording during electroshocks is to determine whether the cerebral electrical activity recorded exclusively or if significant interference from the electrical device, stimulus pulse, muscle activity or movement artifacts has contributed to the recorded signals. In most recordings supply line interference (50Hz AC, Swedish standard) contributed to noise levels when a significant influence was present (2% of recordings) these recording excluded from the analysis. At the reviews a 48Hz cut-off filter used to avoid supply line interference affecting the analyses (Figure 2). In the recorded ECT sessions the stimulus square pulse duration was transformed into frequencies, and it was between 1000-4000Hz frequency with a sampling frequency of 128 Hz, the recording device, therefore could not detect the stimulus square pulse itself but the triggered responses from brain neurons (Figure 1 & 2).
The recorded frequencies did not merely reflect sub-harmonics from the evoked stimulus pulse response because the observed frequencies were not synchronous with the sub-harmonic frequencies, expected from stimulation pulse frequency of 50,60 and 70Hz. The varied frequency responses at the same stimulation pulse frequency also exclude sub-harmonics as an explanation for the observed frequencies in the electroshock recordings (Figure 1 & 3). In some cases, only low frequency (2-3Hz) activity recorded in addition to the stimulus pulse response. Different epileptic peak frequencies were triggered depending on stimulus pulse frequency, and the highest peak frequencies were triggered by the 70Hz pulse stimulus and the lowest by 50Hz pulses. This observation indicates the need for further exploration of the importance of the pulse frequency for effective ECT treatment.
Muscle artifacts: The recordings from the parietal region in the vertex area, distant from the frontal, occipital, and temporal muscles of the skull. With this recording position, it seems unlikely that muscle activity affected recordings to any significant degree. The varying frequency responses, even in the same patient, triggered by electrostimulation also diminish the likelihood that these responses represent a stereotypical muscle response (Figure 1 & 3). Therefore, it appears that the recordings reflect cerebral EEG responses triggered by electrical stimulation during the ECT sessions, especially true for the convulsions in the clonic post-stimulus phase, no high-frequency responses observed (Figure 1).
Epileptic activity: The current view of ECT is that electrical stimulation triggers generalized tonic-clonic epileptic seizures, the tonic part when the electrostimulation is applied and the clonic part of the seizure after electrostimulation aborted. The therapeutic antidepressant effect depends on triggering enough epileptic seizure activity [10,11,17-21]. The responses in this study by the induced epileptic seizure of the electro-shock was an immediate stable epileptic activity with approximately constant amplitude throughout the electrical stimulation (Figure 1 & 2). There was no recruitment or gradually increasing amplitude, as in spontaneous or drug-induced seizures [5,15,22] or the rare “epileptic recruitment rhythm” of bilateral synchronous 18-22Hz EEG activity observed by Weinar [23] was seen in any of the recordings. After electrostimulation aborted, the epileptic tonic activity was replaced by epileptic clonic activity with a mainly low-frequency distribution (Figure 1). The triggered high-amplitude hyper-synchronous epileptic activity with frequency peaks in the Delta to Gamma frequency bands of specific frequencies likely reflects a limitation of cerebral neurons and neural networks to respond and seem dependent of stimulation pulse frequency (Figure 1 & 2). Also corroborated by different peak frequencies dominated in different recordings, but other frequency peaks, dominated in other recordings also was present with varying lower amplitudes apart from the dominant peak frequency, especially when a 70Hz stimulation pulse frequency used (Figure 1 & 2).
Post-stimulus EEG: The disadvantage of using post-stimulus EEG parameters to estimate the efficacy of stimulation due to lack of, or very weak correlation with triggered epileptic activity during the electroshock. Extensive attempts have tried to correlate the post-stimulus parameters and antidepressant treatment effects. The post-stimulus duration showed no correlation in some studies, while other studies claimed either a positive or inverse relationship to the antidepressant treatment effects [11]. The postictal suppression has been reported to be inversely correlated with treatment effects based on either visual or digital EEG analyses [11,12,20] and mid-ictal post-stimulus amplitude associated with good treatment effects [9-11]. To use post-stimulus EEG parameters, seem questionable to value the effect of the delivered electric shock and antidepressant effect. In this study, 13% of the post-stimulus recordings had to be rejected due to movement or supply line interference, even though subdermal needle electrodes used. In comparison, only 2% of the recordings rejected due to supply line interference during the electro-shock stimulation. Another problem of post-stimulus EEG at ECT is the anesthesia drugs antiepileptic effects which may affect post-stimulus EEG parameters [24].
Conclusion
The method of recording and analyzing EEG signals during electrical stimulation presented here may offer improved possibilities to evaluate the responses of triggered electro-shocks in ECT sessions better than post-stimulus EEG parameters. This methodology may lead to more effective optimization of stimulation for patients and treatment results. Moreover, this methodology may also offer opportunities to evaluate the efficacy of different stimulation techniques. No conclusions could be drawn from this study concerning the relationship of the antidepressant effect and epileptic activity triggered by electric shocks. However, other studies shown that a more effective antidepressant treatment result achieved with increasing stimulation intensity [17-21] and a dose-response relationship for the antidepressant effect observed for right unilateral stimulation [11,21]. Further studies are required to investigate the relationship between antidepressant treatment effects and epileptic activity during stimulation at ECT.
Acknowledgment
I thank Håkan Odeberg MD (Psychiatry) and Bo Sköld MD (Anesthesiology) for their valuable help with the study recordings. No grants obtained for this study.
References
- Cerletti U, Bini L (1938) Un nuevo metodo di shockterapie “ L elettroshock”. Bolletino Accademia Medica Roma 64: 136-138.
- Thompson J, Weinar R, Myers C (1994) Use of ECT in the united states in 1975,1980 and 1986. Am J Psychiatry 151(11): 1657-1661.
- Abrams R (2002) Efficacy of electroconvulsive therapy. In: Abrams R (Ed.), Electroconvulsive Therapy, 4th edn, Oxford University Press, Oxford, Great Britain, UK, pp. 17-22.
- Penfield W, Jasper H (1954) Electrophysiology and experimental epilepsy. In: Penfield W, Jasper H (Eds.), Epilepsy and Functional Anatomy of the Human Brain. Little Brown, Boston, USA, pp. 183-238.
- Chatrian G, Peteresen M (1960) The convulsive patterns provoked by Indoklon, Metrazol and electroshock: some depth electrographic observations in human patients. Electroencephalogr Clin Neurophysiol 12: 715-725.
- Small J, Small I, Perez H, Sharpley P (1970) Electroencephalographic and neurophysiological studies of electrically induced seizures. J Nerv Ment Dis 150(6): 479-489.
- Abrams R, Volavka J, Fink M (1973) EEG seizure patterns during multiple unilateral and bilateral ECT. Compr Psychiatry 14(1): 25-28.
- Daniel W, Crowitz H, Weinar R, Swartzwelder H, Kahn E (1985) ECT-induced amnesia and postictal EEG suppression. Biol Psychiatry 20(3): 344-348.
- Nobler M, Sackeim H, Solomou M, Luber B, Devanand D (1993) EEG manifestations during ECT: Effects of electrode placement and stimulus intensity. Biol Psychiatry 34(5): 321-330.
- Krystal A, Weinar R, McCall W, Shelp F, Arias R, et al. (1993) The effects of ECT stimulus dose and electrode placement on the ictal electroencephalogram: an intra-individual cross study. Biol Psychiatry 34(11): 759-767.
- Abrams R (2002) The electroconvulsive therapy stimulus, seizure, induction and seizure quality. In: Abrams R (Ed.), Electronvulsive Therapy. Oxford University Press, Oxford, Great Britain, UK, pp. 101-129.
- Azuma H, Fujita A, Sato K, Arahata K, Otsuki K, et al. (2007) Postictal suppression correlates with therapeutic efficacy for depression in bilateral sine and pulse wave electroconvulsive therapy. Psychiatry and clinical Neuroscience 61(2): 168-173.
- Nyquist H (1928) Certain topics in telegraph transmission theory. Trans IEEE 47(2): 617-644.
- Chatrian G (1974) A glossary of terms commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol 37(5): 538-548.
- Niedermeyer E (2007) Abnormal EEG patterns: epileptic and paroxysmal. In: Niedermeyer E, Silva FLd (Eds.), Electroencephalography, 5th edn, Lippincott, Williams & Wilkins, Philadelphia, USA pp. 255-280.
- Roth M, Kay D, Shaw J, Green J (1957) Prognosis and pentothal induced electroencephalographic changes in electro-convulsive treatment; an approach to the problem of regulation of convulsive therapy. Electroencephalogr Clin Neurophysiol 9(2): 225-237.
- Cronholm B, Ottosson JO (1960) Experimental studies of the therapeutic action of Electroconvulsive Therapy in endogenous depression. Acta Psychiatr Neurol Scand Suppl 35(s145): 69-101.
- Ottosson JO (1960) Experimental studies of the mode of action of electroconvulsive therapy: Introduction. Acta Psychiatr Neurol Scand Suppl 35(145): 5-6.
- Sackeim H, Decina P, Kanzler M, Kerr B, Malitz S (1987) Effects of electrode placement on the efficacy of titrated, low dose ECT. Am J Psychiatry 114(11): 1449-1455.
- Krystal A, Weinar R, Coffey C, Smith P, Arias R, et al. (1992) EEG evidence of more “intense” seizure activity with bilateral ECT. Biol Psychiatry 31(6): 617-621.
- Sackeim H, Prudic J, Devanand D, Nobler M, Lissanby S, et al. (2000) A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psych 57(5): 425-434.
- Penfield W, Jasper H (1954) Electroencephalography. In: Penfield W, Jasper H (Eds.), Epilepsy and Functional Anatomy of the Human Brain. Little Brown, Boston, USA pp. 640-649.
- Weinar RD (1982) Electroencephalographic correlates of ECT. Psychopharmacol Bull 18(2): 78-81.
- Gálvez V, Pavlovic HD, Wark H, Harper S, Leyden J, et al. (2016) The anaesthetic- ECT time interval in electroconvulsive therapy practice-is it time to time? Brain Stimulation 9(1): 72-77.
© 2019 Ekedahl RG. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






