- Submissions

Full Text
Advancements in Case Studies
Toxic Encephalopathy following Methamphetamine, MDMA, and Marijuana Intoxication: A Case Report
Stephen Howell1*, Steven Wright1, Jason Chang2 and Forshing Lui1
1California Northstate University College of Medicine, USA
2Kaiser Permanente Medical Center, USA
*Corresponding author: Stephen Howell, California Northstate University College of Medicine, Elk Grove, USA
Submission:July 14, 2022;Published: July 21, 2022

ISSN 2639-0531Volume3 Issue3
Abstract
Case Description: We present a case where chronic use of MDMA and marijuana present symptoms of encephalopathy, but do not match with imaging, symptom, and testing for single methamphetamine use.
Conclusion: Additional testing and research is needed to determine the effects of combination illicit drug use, specifically marijuana and MDMA to appropriately map areas of damage in the brain. In a patient presenting with symptoms of encephalopathy, with chronic use of both MDMA and marijuana, confounding results are found on symptom review, physical exam, and imaging studies. Further study is warranted to determine the combined effects of illicit drugs on damage to the brain.
Keywords:Case report; Toxic encephalopathy; Marijuana; MDMA; Ataxia
Abbreviations:ADTE=Acute Diffuse Toxic Encephalopathy; CTE=Chronic Toxic Encephalopathy; MDMA=3,4-Methylenedioxymethamphetamine; EEG=Electroencephalography; CT=Computed Tomography; MRI=Magnetic Resonance Imaging; PET=Positron Emission Tomography; MRS=Magnetic Resonance Spectroscopy; fMRI=Functional Magnetic Resonance Imaging; SPECT=Single-Photon Emission Computed Tomography; HIV=Human Immunodeficiency Virus; SARS Cov-2 NAA=Severe Acute Respiratory Syndrome Coronavirus 2 Nucleic Acid Amplification; MDA=Methylenedioxyamphetamine; THC=Tetrahydrocannabinol; DNA=Deoxyribonucleic Acid
Introduction
Toxic encephalopathy is a brain dysfunction secondary to toxin exposure [1]. Toxin exposure is dose-dependent with longer duration of exposure leading to higher probability of permanent consequences [2]. Symptoms present as symmetrical or non-focal, similar to metabolic encephalopathy [2]. Due to the limited ability for the nervous system to heal compared to other organs, damage often leads to long term effects. Acute vs chronic exposure can lead to different presentations of symptoms depending on the toxin. Toxins also prevent delaying of senescence in aging by decreasing functional reserves [2]. While toxic encephalopathy typically occurs in occupations with use of organic solvents, it is also seen with illicit drug use [2]. ADTE is rapid onset over days or weeks of global cerebral dysfunction often associated with decreased levels of consciousness. CTE is slower onset over months or years with different levels of cognitive impairment. CTE is graded I-III or 1, 2A, and 3 depending on symptoms. I, 1, and 2A have changes in memory, mood and concentration. II and 2B have attention and memory deficits that are measurable, learning deficits, and diminished psychomotor function. III and 3 have neurological deficits with associated changes in imaging, specifically brain atrophy or lack of other neurological etiology. The main treatment for CTE is removal of the offending toxin with little reversal of brain damage depending on grade of CTE [2]. Cocaine, amphetamines, including methamphetamine, MDMA, and amyl or butyl nitrites are the most abused psychoactive drugs, also known as “uppers.” Each drug and combination of drugs have different degrees of risk for encephalopathy. Risk for encephalopathy increases with comorbid environmental toxins and nutritional deficiency.
When evaluating a patient for toxic encephalopathy it is important to elicit a thorough history including living situation, occupational history, substance use, travel and hobbies. Exposure to toxins can occur in any aspect of life. A head-to-toe physical exam following a critical history will further narrow down the differential diagnosis for a patient experiencing toxic encephalopathy. Some toxins may be tested for in blood or urine, include but are not limited to lead, mercury, methamphetamine, and MDMA. Objective lab studies can be applied to neurological deficits using neurobehavioral testing. Non-specific diffuse slowing on EEG can help support a diagnosis of toxic encephalopathy. CT and MRI and functional imaging such as PET, MRS, fMRI, and SPECT can further help in the diagnosis of toxic encephalopathy particularly in CTE. In ADTE, MRI shows damage in bilateral basal ganglia or diffuse areas of edema [2]. In CTE, MRI has nonspecific changes with slight brain atrophy [2].
The following case report describes a patient with chronic use of MDMA and marijuana presenting with symptoms of encephalopathy.
Case Narrative
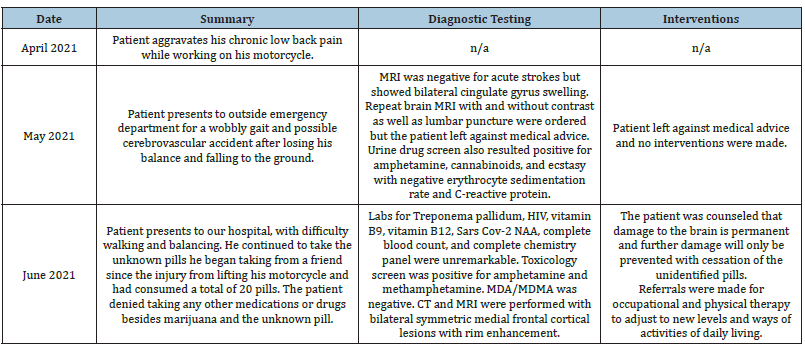
A 61-year-old man with current and chronic tobacco and marijuana use with chronic low back pain presented to the emergency department for “problems walking” for 3 weeks. Two months prior to admission, he aggravated his chronic low back pain while lifting his Kawasaki motorcycle. He mentions a dislike for seeking medical attention when in pain, and instead uses marijuana to help with his lower back pain. However, after lifting the motorcycle, his pain was not alleviated by marijuana, and a friend offered pills to help with the pain. He did not ask what the pills were. The pills eased his pain, gave him more energy, made him feel “productive”, and enabled him to move heavy objects in his garage.
One week prior to admission, he presented to an outside emergency department for a wobbly gait and possible cerebrovascular accident after losing his balance and falling to the ground. He was unable to get up for approximately 15 minutes after which he was able to stand up without assistance. MRI was negative for acute strokes but showed bilateral cingulate gyrus swelling. Repeat brain MRI with and without contrast as well as lumbar puncture were ordered but the patient left against medical advice. Urine drug screen also resulted positive for amphetamine, cannabinoids, and ecstasy with negative erythrocyte sedimentation rate and C-reactive protein.
On admission to our hospital, the patient presented with difficulty walking and balancing. He has been using a cane, but still finds walking and staying balanced difficult. He continued to take the same unknown pills since the injury from lifting his motorcycle and had consumed a total of 20 pills. The patient denied taking any other medications or drugs besides marijuana and the unknown pill. When asked if the friend who supplied the pills might use illicit drugs, the patient responded “yes, he does.” Review of systems positive for some “cracking” with neck movement but no pain and usually chronic low back pain but none today and negative for headaches, fever, chills, neck stiffness, visual changes, nausea, vomiting, bowel or bladder dysfunction, numbness, weakness, hallucinations, memory problems, personality changes, and no other pain.
Physical exam was unremarkable aside from mild hip flexor weakness and severe truncal/gait ataxia without nystagmus or extremity ataxia. Labs for Treponema pallidum, HIV, vitamin B9, vitamin B12, Sars Cov-2 NAA, complete blood count, and complete chemistry panel were unremarkable. Toxicology screen was positive for amphetamine and methamphetamine. MDA/MDMA was negative. CT and MRI were performed shown in Figure 1&2. The treatment plan consisted of counseling the patient with the info that damage to the brain is permanent and further damage will only be prevented with cessation of the unidentified pills. Referrals were made for occupational and physical therapy to adjust to new levels and ways of activities of daily living. The expected outcome is for the patient to stop taking the unidentified pills and participate in occupational and physical therapy. He will have long-term neurocognitive deficits, but deficits will not increase rapidly. The patient was lost to follow up (Table 1).
Figure 1:CT with contrast showing bilateral symmetric medial frontal cortical lesions with rim enhancement.
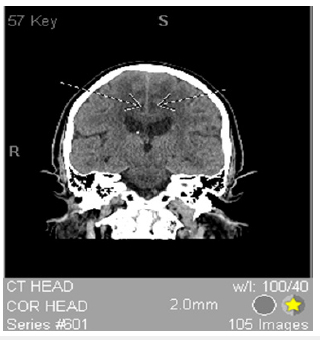
Figure 2: T1 FSE + GAD showing bilateral symmetric medial frontal cortical lesions with rim enhancement.
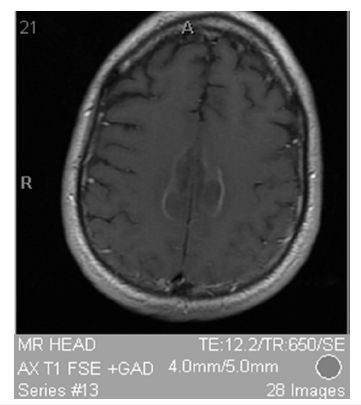
Table 1: Timeline of patient’s clinical course.
Discussion
The degree of encephalopathy related to toxic exposure is dose and duration dependent [2]. Individual drugs have different effects than combination of drugs and even different batches of the same drug can cause different effects due to impurities found in illicit drugs that are unregulated. The general pathophysiology for drug-induced neurotoxicity is lowered antioxidants, increased excitotoxicity, and reduced energy production.
The patient has a history of chronic marijuana and subacute MDMA use. Repeated and prolonged use of these drugs can cause far reaching health issues, specifically encephalopathy with MDMA use. Dopamine is involved in the pleasure and reward centers of the brain [3]. He phenethylamines which include methamphetamine, MDA and MDMA work to increase dopamine in the brain [4]. MDMA use is usually chronic due to sense of euphoria followed by a calm feeling after use [5]. Long term use is associated with brain damage mainly on the striatum and amygdala [6]. Marijuana specifically THC acts through cannabinoid receptors also stimulating the pleasure centers of the brain by indirectly increasing dopamine through inhibiting GABAergic receptors [7]. Marijuana specifically THC has been shown to cause changes in the hippocampus affecting learning and memory [8]. MDMA can lead to nerve cell and terminal degeneration most likely from globally reduced glucose metabolism in the brain [9]. THC in marijuana has been shown to induce apoptosis in cortical neurons by releasing cytochrome C, cleaving DNA repair enzymes, and activating caspase-3 [10].
Toxic encephalopathy usually causes symmetric and widespread damage to the deep gray nuclei and cerebral cortex [11]. MDMA damage is seen in the occipital cortex and globi pallidi with necrosis from prolonged vasospasm [12]. Marijuana damage on imaging is found with decreased cerebral blood flow to all lobes with the mechanisms of vasospasm and vasculitis [12].
The patient’s presentation of mild proximal lower extremity weakness and severe truncal/gait ataxia without extremity ataxia or nystagmus indicates that his ataxia is not cerebellar but frontal. He has “poor truncal mobility, impaired postural responses, and falls after the slightest perturbation [that] make walking impossible even though simple leg movements may still be possible while seated or supine [13]. Frontal gait syndrome is defined by one of these features on initial presentation due to damage to the frontal cortex [13]. The patient most likely has frontal gait syndrome due to damage of his frontal cortex from chronic marijuana and subacute MDMA use. CT and MRI show bilateral symmetric medial frontal cortical lesions with rim enhancement seen in Figure 1&2 confirming damage to the frontal cortex. MDMA use is known to damage the medial frontal cortex [14]. Finally, marijuana use was found to have hypoactivity of the medial frontal cortex with a reduction in gray matter in the medial temporal cortex with long-term use suggesting an additive effect on frontal cortex degeneration [15,16].
Conclusion
Imaging findings with patient endorsed drug use and positive urine drug screens combined with physical exam findings of truncal ataxia and ataxic gait in the absence of extremity ataxia or nystagmus are consistent with changes in brain structure and chronic vascular damage to the frontal cortex from MDMA use. In addition, overlying chronic marijuana most likely compounded damage to the prefrontal cortex by MDMA use and added a generalized decrease in matter in all lobes. It is important to distinguish ataxia with thorough history, physical and appropriate studies. The variability in toxicology results between the outside hospital and that seen in our department are likely due to a combination of variability of concentration within the pills, as well as cross-reactivity between detected substances in the urine toxicology screen. The patient unfortunately was lost for any follow up testing. Additional studies determining the effects of combination drug use, specifically marijuana and an additional illicit drug will need to be performed to appropriately map areas of damage in the brain especially with increasing rates of marijuana use in young adults [17].
Author contributions
Stephen Howell participated in the literature review, drafting, and submission of the manuscript.
Steven Wright participated in the literature review, drafting, and submission of the manuscript.
Jason Chang participated in the data collection, critical review, and submission of the manuscript.
Forshing Lui participated in critical review and submission of the manuscript.
References
- Firestone J, Longstreth W (1994) Textbook of clinical occupational and environmental medicine.
- Kim Yangho, Jae Woo Kim (2012) Toxic encephalopathy. Safety and health at work 3(4): 243-256.
- Oscar AC, Stamelou M, Rodriguez EM, Gonalez MM, Poppel E (2010) Dopaminergic reward system: a short integrative review. International archives of medicine 3: 24.
- Gouzoulis-Mayfrank, E, Schreckenberger M, Sabri O, Arning C, Thelen B, et al. (1999) Neurometabolic effects of psilocybin, 3,4-methylenedioxy- ethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo- controlled PET study with [18F] FDG. Neuropsychopharmacol 20(6): 565–581.
- Virmani Ash, Schmued Larry, Gaetani F, Ali Syed, Binienda Z (2008) Encephalopathies related to abuse of psychoactive drugs. Drug Related Encephalopathies, pp. 227-260.
- Buchert R, Obrocki J, Thomasius R, Väterlein O, Petersen K, et al. (2001) Long-term effects of ‘ecstasy’ abuse on the human brain studied by FDG PET. Nucl Med Commun 22(8): 889-897.
- NIDA (2021) How does marijuana produce its effects?
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, et al. (2009) Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19(8): 763-772.
- Downer Eric, Boland B, Fogarty M, Campbell (2001) Delta 9-Tetrahydrocannabinol induces the apoptotic pathway in cultured cortical neurons via activation of the CB1 receptor. Neuroreport 12(18): 3973-3978.
- Sharma P, Eesa M, Scott JN (2009) Toxic and acquired metabolic encephalopathies: MRI appearance. AJR Am J Roentgenol 193(3): 879-886.
- Tamrazi, Benita, Jeevak Almast (2012) Your brain on drugs: imaging of drug-related changes in the central nervous system. Radio graphics 32(3): 701-719.
- Thompson PD (2012) Frontal lobe ataxia. Handb Clin Neurol 103: 619-622.
- Pan HS, Wang RY. (1991) The action of (+/-)-MDMA on medial prefrontal cortical neurons is mediated through the serotonergic system. Brain Res 543(1): 56-60.
- Gamma A, Buck A, Berthold T, Liechti ME, Vollenweider FX (2000) 3,4-Methylenedioxymethamphetamine (MDMA) Modulates Cortical and Limbic Brain Activity as Measured by [H215O]-PET in Healthy Humans. Neuropsychopharmacol 23: 388-395.
- Wesley MJ, Lile JA, Hanlon CA, Porrino LJ (2016) Abnormal medial prefrontal cortex activity in heavy cannabis users during conscious emotional evaluation. Psychopharmacology (Berl) 233(6): 1035-1044.
- Battistella, Giovanni, Fornari E, Annoni JM, Chtioui H, Dao K et al. (2014) Long-term effects of cannabis on brain structure. Neuro psychopharmacology 39(9): 2041-2048.
- Marijuana use at historic high among college-aged adults in 2020. National Institutes of Health, U.S. Department of Health and Human Services.
© 2022Stephen Howell. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






