- Submissions

Full Text
Advancements in Case Studies
Adult-Onset MELAS in a 28-year-old Female Presenting with Visual Loss and Type 1 Diabetes Mellitus: A Case Report
Aleeha Noon1*, Lillian Jundi1*, John Geraghty2 and Forshing Lui1
1California North state University College of Medicine, USA
2Kaiser Permanente Roseville Medical Center, USA
*Corresponding author: Aleeha Noon and Lillian Jundi, California North state University College of Medicine, USA
Submission:May 27, 2022;Published: June 13, 2022

ISSN 2639-0531Volume3 Issue3
Abstract
Purpose: To present an unusual case of MELAS with initial symptomatic onset in a 28-year-old female
recently diagnosed with type 1 diabetes mellitus.
Introduction: Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS)
is a mitochondrial DNA inherited disorder that presents primarily with neurologic symptoms, such as
visual loss migraine-like headaches and stroke like episodes not corresponding to any specific vascular
territory. Most cases of MELAS present in childhood or before the age of 20. We present a case of MELAS
with initial presentation at 28 years old in a patient with recently diagnosed type 1 diabetes mellitus with
medication noncompliance.
Case summary: A 28-year-old Caucasian right-handed female presented to the emergency room with
sudden-onset occipital headache and vision loss. In the ER, she experienced a tonic-clonic seizure
responsive to lorazepam. Neurological exam revealed left homonymous hemianopsia. Neuroimaging
found evidence of a right inferior temporal/occipital lesion, initially thought to be consistent with
posterior reversible encephalopathy syndrome. Repeat imaging and further laboratory workup was
indicated to evaluate for metabolic etiologies given her persistence of symptoms and diabetes history.
Serum lactate was then found to be markedly elevated. A molecular genetics report demonstrated that the
patient was hetero-plasmic in the MT-TL1 gene (37.6%) for a sequence variant designated m.3243A>G.
These results confirmed the final diagnosis of MELAS.
Conclusion: Though rare, MELAS may present in adulthood, as exhibited in this patient. In these cases,
measurement of lactate and genetic studies significantly contribute to the final diagnosis. Furthermore,
concurrent metabolic comorbidities such as recently diagnosed type 1 diabetes strengthen the diagnostic
consideration for MELAS.
Keywords: MELAS; Lactic acidosis; MT-TL1gene
Abbreviations:MELAS: Mitochondrial Encephalopathy with Lactic Acidosis and Stroke-like Episodes; CSF: Cerebrospinal Fluid; PRES: Posterior Reversible Encephalopathy Syndrome; tRNA: Transfer RNA
Introduction
Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) is a mitochondrial DNA inherited condition primarily affecting the nervous and musculoskeletal systems. It can present in a sporadic or maternal inherited pattern. Consequently, the clinical presentation of MELAS is variable but typically presents with neurologic events such as stroke-like episodes, headache, epilepsy, and dementia. Occasionally, MELAS may involve other organ systems including endocrine (e.g., diabetes), cardiovascular (e.g., cardiomyopathy), and gastrointestinal (e.g., anorexia) [1,2]. The current diagnostic guidelines for MELAS require the patient to meet at least two criteria each in two general categories [3]. The first category of criteria involves the clinical manifestations of the disease, including the presence of seizures, hemiplegia, headaches associated with vomiting, cortical blindness (or hemianopsia), and lesions on neuroimaging [4]. The second category of criteria is in regard to evidence of mitochondrial dysfunction, including elevated CSF or plasma lactate, muscle biopsy indicative of abnormalities of the mitochondria, and/or the possession of a gene associated with MELAS [4]. This study presents a case of MELAS with initial presentation at 28 years old in a patient with recently diagnosed type 1 diabetes mellitus.
Case Presentation
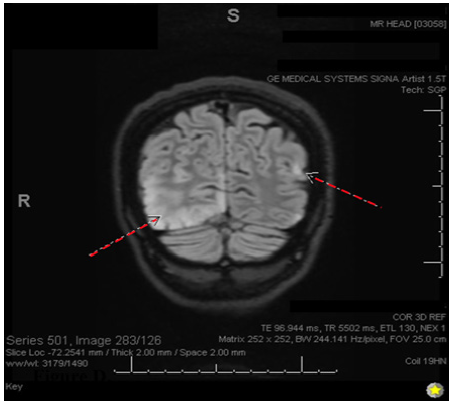
A 28-year-old Caucasian right-handed female who presented to the emergency room with complaints of sudden-onset occipital headache and loss of vision. The patient says that she had been sorting through paperwork when she suddenly felt the headache and began seeing dark spots in her vision. In the emergency room, she was witnessed to have a seizure in which her eyes rolled upwards, her head deviated to the left, and her body made jerky movements on the left side without any evidence of tongue biting or loss of bowel/bladder control. At the time, the patient responded well to 1mg lorazepam given IV, and she had relatively prolonged post-ictal confusion. The patient was admitted for neurological workup and observation. Throughout her admission, she remained very somnolent and tearful, and she was still in significant pain with her headache. She describes her headache as 10/10 intensity and throbbing, with lateralization to the right side. She says she cannot see on her left side as well, so she keeps her eyes closed. She is also very nauseous and has been vomiting minimal, green-tinted emesis periodically. The patient says she also recently lost 10 pounds over a few months unexpectedly. She denies any chest pain, abdominal pain, fevers, chills, dysuria, or diarrhea (Figure 1-3).
Figure 1: Computed Tomography (CT) scan of the head. Showing vague hypodensity in the right parietal lobe (indicated by dashed red arrow).
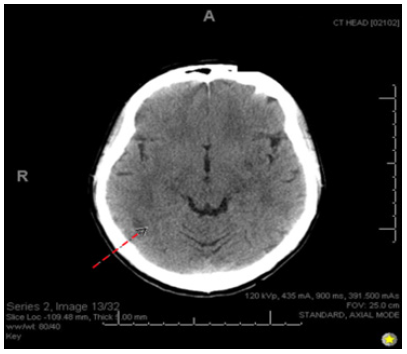
Figure 2: Diffusion-Weighted Image (DWI). Showing diffusion restriction with the ADC map in both hemispheres with right > left prominence.
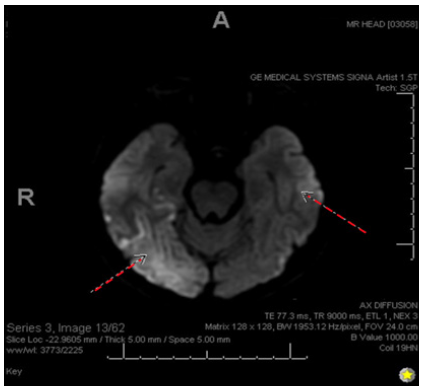
Figure 3: Coronal 3D fluid-attenuated inversion recovery (FLAIR) showing FLAIR hyperintensity in both hemispheres (R>L).
On examination, the patient appeared somnolent but alert & oriented to person, place, and time with fluent speech. Neurological examination revealed left homonymous hemianopia with normal extraocular movements and pupillary responses. Cranial nerve examinations were normal. Motor strength was 5/5 in all extremities. Examination of reflexes showed +2 reflexes in her upper extremities bilaterally but hyperactive in her lower extremities with sustained ankle clonus bilaterally. Plantar responses were flexor bilaterally. Sensation to pinprick and light touch were intact. Finger-to-nose testing and heel-to-shin testing were normal.
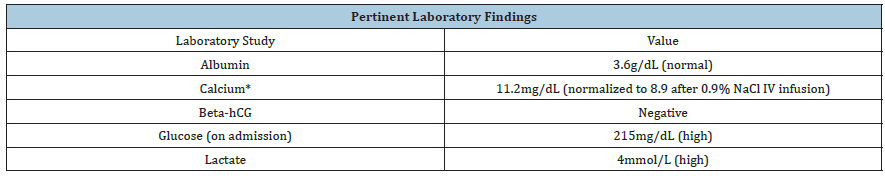
Based on the initial clinical picture, the transient hypercalcemia, and neuroimaging findings, the patient was diagnosed with posterior reversible encephalopathy syndrome (PRES). Further review of her clinical picture and images suggested the etiology to be inconsistent with PRES but more likely to be secondary to an underlying toxic or metabolic disturbance. Thus, a serum lactate was obtained and found to be markedly elevated (Table 1).
Table 1: *PTH, SPEP, ACE, and vitamin D levels were also drawn to work up the cause of hypercalcemia, but each of these studies was found to be within normal range.
A subsequent molecular genetics report demonstrated that the patient was heteroplasmic in the MT-TL1 gene (37.6%) for a sequence variant designated m.3243A>G. This is one of the commonest mitochondrial DNA mutations. Common reported phenotypes may include mitochondrial myopathy, encephalopathy, lactic acidosis and stroke like episodes (MELAS), chronic progressive external ophthalmoplegia (CPEO), and meternally inherited deafness and diabetes (MIDD). MELAS is the most common phenotype of this mutation.
Treatment and Outpatient Follow Up
The patient’s current treatment plan is multi-faceted. Her seizures are currently controlled with Lamotrigine 150mg twice a day and Lacosamide 100mg twice a day. The patient has resumed treatment for her type 1 diabetes with glipizide 5 mg and insulin. Additionally, she is supplementing her medications with coenzyme Q10 600mg daily, citrulline 5gms/ft2 divided three times a day, arginine 10g/m2, and Taurine 500mg twice a day.
Discussion
Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) is a mitochondrial DNA inherited condition with a wide array of clinical presentations and symptoms [1]. These presentations can include neurologic events such as stroke-like episodes, epilepsy, and dementia or may involve many other systems including endocrine (diabetes), cardiovascular (cardiomyopathy), and gastrointestinal [1]. The more current diagnostic guidelines for MELAS require the patient to meet at least two criteria each in two general categories [3]. The first category of criteria involving clinical manifestations of the disease includes: seizures, hemiplegia, headaches associated with vomiting, cortical blindness (or hemianopsia), and focal lesions on neuroimaging [4]. The second category of criteria involving mitochondrial dysfunction includes: Elevated CSF or plasma lactate, muscle biopsy indicative of abnormalities of the mitochondria, and the possession of a gene associated with MELAS [4]. The patient discussed in this case study met both categories of criteria. Clinically, she presented with a seizure, headaches, hemianopsia and diagnostically, her laboratory findings showed elevated serum lactate levels and a mutation in the MT-TL1 gene. MELAS is most commonly caused by a mutation in the mitochondrial tRNA at m.3243A>G, which is found in about 80% of patients with the condition [2]. Other less common but related mutations include the MT-TL1 gene: m.3271T>C or m.3252A>G and the ND5 gene: m.13513G>A [5].
The initial presentation of MELAS in patients typically occurs after age 2 but before age 40, with up to 76% of presentations occurring before 20 years of age [3]. Our patient’s presentation, thus, is very unique as she initially presented at 28-years-old. She has no prior history of seizures or other neurological findings. Given that MELAS has a metabolic component to its pathogenesis, it is notable that our patient was newly diagnosed with type 1 diabetes mellitus, which is also a metabolic disorder. Type 1 diabetes typically presents in early childhood to early adolescence, with a bimodal peak between 4-7 years and 10-14 years [6]. The clinical presentation of two metabolic disorders relatively late in onset brings forth the question whether there is a relationship between these metabolic disorders and potentially the MT-TL1 gene mutation. A case report from 1995 recounts a 32-year-old female patient who presented with MELAS, diabetes mellitus, and hyperthyroidism-three concurrent metabolic pathologies [7]. This patient also had an A-to-G transition at the 3243rd nucleotide position in the MT-TL1 gene. A key difference is the mutation load; our patient demonstrates 37.6% heteroplasmy while the patient in the referenced case report presented with 60% heteroplasmy in the MT-TL1 gene [7]. The increased genetic mutation load in the latter patient may have contributed to the development of a third metabolic disorder. The relatively lower mutational load in this patient was considered as a contributing factor to her late onset presentation. However, the patient in the comparison case study also presented late, at age 25, despite retaining a mutation load nearly double that of our patient.
A retrospective study on DNA mutational load of 57 children with MELAS found that while the mutation load in the MELAS may be associated with the onset of symptoms and associated diseases (like diabetes), it is not known to influence disease progression [8]. Specifically, this study showed an inverse relationship between mutational load and age of onset, such that earlier onset correlates with a higher DNA mutational load [8]. However, progression of an associated disease (i.e., rates of change in insulin sensitivity over time) did not progress in the same regression [8]. Thus, there is a theorized explanation for our patient’s late onset MELAS but not when compared to the other referenced case report. This sparks the question regarding how much DNA mutational load contributes to the overall clinical presentation for the patient, including age of onset, associated diagnoses, and presenting symptoms. Since there is so much variability between cases of MELAS, it is difficult to pinpoint the exact causes and predispositions for each presentation. More research is needed in order to better understand the pathophysiology of MELAS in regard to the heterogeneity of its presentation. In an effort to provide more clinical data points, this case report seeks to exemplify how a thorough diagnostic workup of a patient presenting with concurrent metabolic disease and neurological symptoms may be in fact presenting with MELAS, despite the non-classical age of onset.
Conclusion
Though rare, MELAS may present in adulthood, as exhibited in this patient. In these cases, measurement of lactate and genetic studies significantly contribute to diagnosis. Furthermore, concurrent metabolic comorbidities such as recently diagnosed type 1 diabetes strengthen the clinical suspicion for MELAS. This case seeks to demonstrate the utility of a thorough diagnostic workup including neuroimaging and lactate levels, in the diagnosis of MELAS and its association with a history of new-onset metabolic disease and new-onset neurological symptoms.
Conclusion
Aleeha Noon, participated in the literature review, drafting, and submission of the manuscript.
Lillian Jundi, participated in the literature review, drafting, and submission of the manuscript.
John Geraghty, participated in the data collection, critical review, and submission of the manuscript.
Forshing Lui, participated in the critical review and submission of the manuscript.
References
- El-Hattab AW, Adesina AM, Jones J, Scaglia F (2015) MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab 116(1-2): 4-12.
- Niedermayr K, Pölzl G, Scholl-Bürgi S, Fauth C, Schweigmann U, et al. (2018) Mitochondrial DNA mutation "m.3243A>G"-Heterogeneous clinical picture for cardiologists ("m.3243A>G": A phenotypic chameleon). Congenit Heart Dis 13(5): 671-677.
- El-Hattab AW, Almannai M, Scaglia F (2001) MELAS. In: Adam MP, Ardinger HH, Pagon RA, et al., (Eds.), Gene Reviews®. Seattle (WA): University of Washington, Seattle; 1993-2022.
- Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, et al. (2012) MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta 1820(5):619-624.
- Ikeda T, Osaka H, Shimbo H, Tajika M, Yamazaki M, et al. (2018) Mitochondrial DNA 3243A>T mutation in a patient with MELAS syndrome. Hum Genome Var 5: 25.
- Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, et al. (2018) Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 6(2): 122-129.
- Yang CY, Lam HC, Lee HC, Wei YH, Lu CC, et al. (1995) MELAS syndrome associated with diabetes mellitus and hyperthyroidism: A case report from Taiwan. Clin Endocrinol (Oxf) 43(2): 235-239.
- Chae HW, Na JH, Kim HS, Lee YM (2020) Mitochondrial diabetes and mitochondrial DNA mutation load in MELAS syndrome. Eur J Endocrinol 183(5): 505-512.
© 2022 Aleeha Noon and Lillian Jundi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






