- Submissions

Full Text
Advancements in Case Studies
Heidenhain Variant Creutzfeldt-Jakob Disease Identified with RT-QuIC Assay: A Case Report
Gavin Zanella1*, Nicholas Baltar1*, Forshing Lui1, Ning Zhong2,3,4 and Kaho Wong5
1California Northstate University College of Medicine, USA
2Kaiser Permanente North Valley Epilepsy Program, USA
3Kaiser Permanente Northern California Regional Epilepsy Center, USA
4Department of Neurology, Kaiser Permanente Sacramento Medical Center, USA
5Department of Neurology, Kaiser Permanente Roseville Medical Center, USA
*Corresponding author: Gavin Zanella and Nicholas Baltar, California Northstate University College of Medicine, USA
Submission:January 14, 2022;Published: January 21, 2022

ISSN 2639-0531Volume3 Issue3
Abstract
Background: Heidenhain variant Creutzfeldt-Jakob disease (HV-CJD) is a form of spongiform
encephalopathy characterized by isolated visual symptoms that rapidly progress through a series of often
non-specific CNS symptoms that ultimately result in severe dementia, myoclonus, and radically altered
mental status. Due to the non-specific nature of this disease’s progression, early diagnosis is challenging
and can place large amounts of stress and uncertainty on the patient’s loved ones.
Case Description: We present the case of a 61-year-old female who arrived at the clinic with complaints
of isolated bilateral visual disturbances, which resulted in a diagnosis of occipital ischemic stroke after
MRI was performed. Weeks later, she rapidly progressed from focal seizures to non-convulsive status
epilepticus refractory to treatment with anticonvulsants and benzodiazepines. At this time, she was
diagnosed with Creutzfeldt-Jakob disease (CJD) using a relatively new diagnostic test called “real time
quaking induced conversion” (RT-QuIC).
Conclusion: Before the advent of the RT-QuIC assay, the diagnosis of CJD was complicated by the lack
of a sensitive and specific test and the absence of a uniform clinical course. This case should indicate
to readers that the RT-QuIC assay is a high sensitivity and specificity test available for the diagnosis of
suspected CJD.
Keywords:Hv-CJD; Creutzfeldt-Jakob disease; RT-QuIC, Case report; Neurology; Ophthalmology
Abbreviations:CJD=Creutzfeldt-Jakob disease; CSF=Cerebrospinal Fluids; sCJD=Spontaneous Creutzfelt- Jakob Disease; DWI=Diffusion Weighted Imaging; EEG=Electroencephalogram; GPD=Generalized Periodic Discharges; Hv-CJD=Heidenhain Variant Creutzfeldt-Jakob Disease; MRI=Magnetic Resonance Imaging; NCSE=Non-convulsive Status Epilepticus; PrPC=Normal Prion Proteins; PrPSC=Pathological Prion Protein; RT-QuIC=Real Time Quaking Induced Conversion; vCJD=Variant Creutzfedlt-Jakob Disease
Introduction
Creutzfeldt-Jakob Disease (CJD) is a rare rapidly progressive neurodegenerative disease caused by misfolded prion proteins. Sporadic CJD (sCJD) accounts for approximately 85% of CJD cases commonly presenting with rapidly progressing dementia, myoclonus, cerebellar and extra-pyramidal signs and visual disturbances [1]. A subset of sCJD patients initially present with exclusively visual abnormalities and develop neurocognitive defects later in the disease course. This is termed the Heidenhain variant (HvCJD) which likely accounts for approximately 5% of sCJD cases [2]. This variant is often misdiagnosed before progression of further neurological symptoms [3].
Case Narrative
A 61-year-old right-handed female with a past medical history of localized amyloidosis, patent foramen ovale and genital HSV presented with complaints of new onset diminished and blurry vision bilaterally, image distortion, and difficulty focusing on objects. She denied eye pain, new headaches, and other neurological symptoms. Neurological exam was unremarkable, except visual field testing revealing homonymous right inferior quadrantic hemianopia and MRI showed left occipital diffusion restriction (Figure 1A). This led to an initial diagnosis of acute left posterior cerebral infarction. The patient returned 3 weeks later with complaints of worsened vision loss and increasing difficulty focusing on objects. She described her vision as being “weird” but could not offer more details. Repeat MRI was stable and repeat visual field testing showed worsening right lower quadrantanopia. The patient was started on valproate due to concern for focal seizure without impaired awareness. One week later, the patient returned with persistent decline in vision that was severely impacting her activities of daily living. She also reported that she was perceiving movement of objects that she knew were stationary and visual disturbances that she likened to those experienced when drinking too much alcohol. Around the same time, her family witnessed waxing and waning mental status accompanied by blank staring spells in the middle of conversations and a decline in short-term memory. Outpatient EEG showed brief slow and spike activity in the left occipital area accompanied by generalized slowing (Figure 2A).
Figure 1: MRI displaying small cortical diffusion restriction indicative of an acute to subacute infarction in the left occipital lobe. Side A displays the earliest MRI taken. Side B displays the latest MRI, performed five weeks after the first. No significant changes were noted on MRI in this timespan
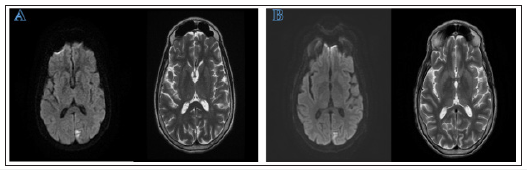
Figure 2: (A) EEG displaying sharp wave complexes. This reading was performed upon first complaint of seizure activity. (B) EEG displaying GPD pattern with 2Hz discharges and a spike component in the posterior regions on bipolar montage.
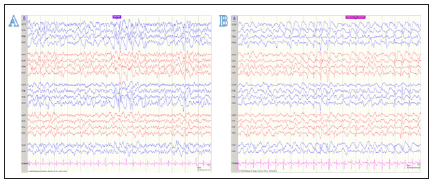
In the next week, the patient showed worsening of her clinical focal seizure activity and was hospitalized. By this time, neurological exam showed partial central vision loss and right lower quadrantanopia. Repeat brain MRI showed stable left occipital signal on DWI without enhancement or worsening of cerebral edema (Figure 1B). Repeat EEG showed epileptiform discharge in the left occipital region and focal seizure activity. Over the following two days, the patient was noted to have continuous decline of consciousness progressing to an inability to follow commands. Repeat EEG at this time showed sustained rhythmic 2-3hz generalized spike-wave activity that was more organized in the left occipital region, raising concern for NCSE (Figure 2B).The patient was transferred to another facility and continued to be non-verbal, restless, and showing signs of altered consciousness. After arrival, exam showed intermittent jerking movement of all extremities lasting 3-5 seconds. cEEG showed NCSE with persistent focal epileptiform discharges in the posterior occipital regions. The patient was started on sedatives and five anticonvulsants, but complete cessation of clinical symptoms or recovery to normal mentality was never achieved.
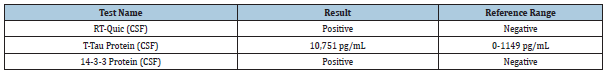
Due to the refractory nature of the patient’s altered mental status and the EEG findings, further workup to explore other etiologies was performed. CSF sample was obtained and showed no evidence of infection and an unremarkable CSF chemistry. CJD and autoimmune encephalitis panels were ordered. No informative auto-antibodies were detected on the extensive autoimmune encephalitis panel. CJD panel was positive for 14-3-3 protein, RTQuic and elevated levels of T-tau protein (Table 1). RT-Quic results indicated a likelihood of prion disease >98%.
Table 1: CJD Panel. CSF analysis revealed elevation of all three markers used to identify CJD. T-Tau and 14-3-3 are non-specific markers of diffuse cerebral damage that are not always present in patients with CJD (4). RT-Quic is the most useful of these measurements, with a reported 92% sensitivity and 100% specificity [4].
The patient was given multiple anticonvulsants and sedatives in an attempt to manage seizure activity, with minor to no improvement throughout the disease course. The patient was also tentatively treated with intravenous steroids and immunoglobulins while awaiting the results of the autoimmune encephalitis panel, which yielded no clinical improvement. After revealing the positive result of the RT-QuIC assay, the patient’s family elected comfort care and the patient expired shortly afterwards. Autopsy was arranged at the National Prion Disease Pathology Research Center.
Discussion
Heidenhain variant Creutzfeldt Jakob Disease has historically been a challenge to diagnose due to the seemingly benign onset being followed by a rapid progression of more concerning, yet often non-specific, symptoms. Physicians may be faced with a patient complaining of something as innocuous as blurred vision who, in a matter of months following the initial complaint, is being hospitalized due to a new onset of rapidly progressive dementia, myoclonus, and radically altered mental status that is refractory to standard treatment [2,4]. In less common presentations such as our patient, physicians may also have to consider abnormal EEG findings, localized MRI findings, and other atypical symptoms whilst sorting through an already broad differential for their quickly ailing patient. To make matters even more complicated, there has not been an exceptionally sensitive or specific lab test for CJD until the recent advent of real time quaking induced conversion (RT-QuIC) testing.
RT-QuIC is a laboratory assay that detects pathological prion protein (PrPSC) using its own ability to convert normal prion proteins (PrPC) into the pathological conformation [5]. This test, which requires only a CSF sample, has been reported to carry a sensitivity of 92% and a specificity of 100% [5]. This represents a marked improvement, particularly in specificity, over the most commonly used surrogate markers for CJD: t-tau and 14-3-3 protein [5-8]. While the typical clinical course and the relative rarity of CJD still make early identification challenging, RT-QuIC offers a feasible route to probable diagnosis before a definitive diagnosis can be made at autopsy [7]. Any physician confronted with a patient complaining of isolated visual symptoms who subsequently develops rapidly progressive dementia along with myoclonus, seizures, or any other neurological symptoms should consider HvCJD and order an RT-QuIC assay.
This case also illustrates that various atypical neuro-imaging findings may present in patients with sCJD. DWI MRI is commonly performed to aid in diagnosis of sCJD. sCJD commonly presents as bilateral symmetric hyperintense cortical or sub-cortical areas, known as a “cortical ribbon” sign [9,10]. In HvCJD, a common radiological feature is a bilateral hyper-intense signal localized primarily to the occipital cortex seen on DWI MRI [10,11]. This is seen in many other cases involving a large area of the occipital cortex [2,11,12]. Additionally, some patients show an expanding area of hyper-intensity eventually infiltrating the parietal cortex [2]. In our case, the patient presents with a stable, localized hyperintense signal in the left occipital cortex. Interestingly, this area of hyper-intensity was not symmetrical and did not cross the midline. This small lesion led to the initial diagnosis of a left occipital ischemic stroke. It is important to note that this sCJD variant can present with atypical MRI findings including focal unilateral lesions. This finding shows how sCJD should be considered as a differential diagnosis even if the MRI findings are atypical.
In contrast to the neuroimaging findings, EEG readings in this case were supportive of a probable CJD diagnosis. The World Health Organization’s diagnostic criteria for CJD cite periodic sharp wave complexes as being supportive of a probable diagnosis [8]. Our patient did not display these changes early in the disease course, but later progressed to GPDs as their seizure activity worsened. Focal seizure activity in the disease course complicated diagnosis because seizures are an uncommon symptom in sCJD patients occurring in less than 15% of patients [9]. This symptom is even less common in HvCJD with a prevalence of 5.5% [13]. Our patient was first hospitalized due to focal seizure activity, which later progressed to NCSE and the patient required sedation. Seizures continued throughout the disease course and proved to be refractory to several medications. Managing seizures in sCJD patients with antiepileptics continues to be extremely challenging [10,12].
Conclusion
The complex nature of this case and many other cases of Heidenhain variant Creutzfeld Jakob disease underscore the importance of using multiple diagnostic modalities in addition to a more traditional history and physical exam. While guidelines for the use of MRI, EEG, and surrogate biomarkers have been in place for many years, none of these modalities are highly specific for CJD and have been known to be negative in cases of CJD confirmed at autopsy [8,14]. While one can wait for pathological confirmation of definite CJD after a patient has expired, this places undue stress and uncertainty on the patient’s family and the care team. The RTQuIC test for CJD should be used to aid in the diagnosis of patients suspected to have CJD, as it is the only highly specific test available at this time [7]. Additionally, the test can be performed at the same time as other CSF assays that a physician may be ordering to rule out other differential diagnoses such as autoimmune encephalitis. The inclusion of such a lab test in the physician’s tool belt is promising for a future in which HvCJD can be more readily diagnosed at an early stage, bringing more certainty to families and physicians alike.
Author Contributions
Gavin Zanella participated in the literature review, drafting, and submission of the manuscript.
Nicholas Baltar participated in the literature review, drafting, and submission of the manuscript.
Forshing Lui participated in critical review and submission of the manuscript.
Ning Zhong participated in the data collection, critical review, and submission of the manuscript.
Kaho Wong participated in data collection.
References
- Appleby ABS, Cohen ML (2021) Creutzfeldt-Jakob disease, pp. 1-45.
- Baiardi S, Capellari S, Ladogana A, Strumia S, Santangelo M, et al. (2016) Re-visiting the heidenhain variant of creutzfeldt-jakob disease: Evidence for prion type variability influencing clinical course and laboratory findings. J Alzheimer’s Dis 50(2): 465-476.
- Cooper SA, Murray KL, Heath CA, Will RG, Knight RSG (2005) Isolated visual symptoms at onset in sporadic Creutzfeldt-Jakob disease: The clinical phenotype of the “Heidenhain variant”. Br J Ophthalmol 89(10): 1341-1342.
- Tsuji Y, Kanamori H, Murakami G, Yokode M, Mezaki T, et al. (2004) Heidenhain Variant of Creutzfeldt-Jakob Disease: Diffusion-weighted MRI and PET characteristics. J Neuro imaging 14(1): 63-66.
- Green AJE (2019) RT-QuIC: A new test for sporadic CJD. Pract Neurol 19(1): 49-55.
- Lattanzio F, Abu-Rumeileh S, Franceschini A, Kai H, Amore G et al. (2017) Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol 133(4): 559-578.
- McGuire LI, Peden AH, Orrú CD, Wilham JM, Appleford, et al. (2012) Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol 72(2): 278-285.
- CDC (2010) CDC’s Diagnostic Criteria for Creutzfeldt-Jakob Disease (CJD). Report of a WHO consultation 9:2.
- Wieser HG, Schindler K, Zumsteg D (2006) EEG in Creutzfeldt-Jakob disease. Clin Neurophysiol 117(5): 935-951.
- Ng MC, Westover MB, Cole AJ (2014) Treating seizures in Creutzfeldt-Jakob disease. Epilepsy Behav Case Reports 2(1): 75-79.
- Schroter A, Zerr I, Henkel K, Tschampa HJ, Finkenstaedt M, et al. (2000) Magnetic resonance imaging in the clinical diagnosis of Creutzfeldt-Jakob disease. Arch Neurol 57(12): 1751-1757.
- Espinosa PS, Bensalem-Owen MK, Fee DB (2010) Sporadic Creutzfeldt-Jakob disease presenting as non-convulsive status epilepticus case report and review of the literature. Clin Neurol Neurosur 112(6): 537-540.
- Muthusamy S, Garg P, Chandra RV, Seneviratne U (2021) How common are seizures in the heidenhain variant of creutzfeldt-jakob disease? A case report and systematic review. J Clin Neurosci 86: 301-309.
- Jacobs DA, Lesser RL, Mourelatos Z, Galetta SL, Balcer LJ (2001) The heidenhain variant of creutzfeldt-jakob disease: Clinical, pathologic, and neuroimaging findings. J Neuro-Ophthalmology 21(2): 99-102.
© 2022 Gavin Zanella. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






