- Submissions

Full Text
Advancements in Case Studies
Case Report: Hyperglycemia-an Ominous Symptom of Disease Course of Covid-19 in Full-term Newborn
Lauterbach R1*, Pawlik D1, Ulatowska Białas M2, Lisowska Miszczyk I1 and Kacprzak M3
1Department of Neonatology, Jagiellonian University Medical College, Poland
2Chair and Department of Patho-morphology, Jagiellonian University Medical College, Poland,
3MEDGEN Medical Centre, Poland
*Corresponding author: Ryszard Lauterbach, Department of Neonatology, Jagiellonian University Medical College, Poland
Submission:July 15, 2021;Published: July 26, 2021

ISSN 2639-0531Volume3 Issue2
Abstract
The first four weeks after birth seem to be a relatively safe period in relation to the first pandemic of the century. Although SARS-CoV-2 can be transmitted from the mother to the newborn through droplets, the clinical symptoms are usually nonsignificant and uncomplicated. It is especially truth when SARSCoV- 2 infection concerns the full-term newborn. We present the fatal disease course of COVID-19 in full term newborn delivered vaginally with appropriate weight and with Apgar score 10. On the fourteenth day of life, neonate rapidly deteriorated and within the next three days died of hyperglycemia that stimulated an inflammatory reaction.
Keywords: SARS CoV-2; COVID-19; Newborn; Hyperglycemia; Inflammatory response
Introduction
The disease course of COVID-19 is usually mild and self-limiting in term newborns, but they may also develop severe symptoms. It is particularly observed in infants with underlying comorbidities. We present a case of 14 days-old full-term newborn, male with birth weight 3680g, delivered in 39th week of pregnancy with Apgar score 10. Previously healthy, he was discharged from hospital on 4th day of life with a 200g weight gain. After 10 days, neonate rapidly deteriorated with poor feeding, effort dyspnea and significantly lowered muscular tonus. Five days prior to acute worsening of child condition, mother reported smell disturbances, but no presence of fever, cough, or nasal congestion.
Clinical findings, diagnostic assessment, and therapeutic intervention
Infant was transported by an ambulance to an emergency department with symptoms
of dehydration, progressive respiratory insufficiency, and loss of consciousness. Moreover,
the weight gain of infant between the discharge from neonatal ward and the admission to
emergency department (10 days) was 100g. Neonatal resuscitation and then intubation and
conventional mechanical ventilation (SIMV) with inspired oxygen fraction titrated up to 0.6-
0.7 was provided. Infant temperature was 37.8 °C, heart rate of 190 beats per minute and
mean blood pressure of 32mmHg. Skin perfusion was significantly disturbed with capillary
refill time above 4 seconds. Initial arterial blood gas analysis showed metabolic acidosis (pH-
7.15, BE (-10,4), lactate 11mmol/L) and an initial point-of-care blood glucose was 185mg/
dL. White and red blood cells as well as platelet count, showed normal values. Other main
laboratory findings in the neonate like C-reactive protein, procalcitonin, liver enzymes
(AST, ALT) and electrolytes concentrations also presented normal values. Point-of-care
echocardiography showed an increased resistance in pulmonary arteries circulation and no
congenital cardiac malformations. In the emergency department the patient received a 20mL/kg normal saline bolus, and the fluid infusion was continued in a
dose of 10ml/kg/h. Furthermore, after cardiologist’s suggestion,
dopamine (5ug/kg/min) and corotrope (0.75ug/kg/min) was
started. On admission to the emergency department, as per current
hospital policy, infant nasopharyngeal swab was tested for the
presence of SARS-CoV-2 RNA and real-time polymerase chain
reaction (RT-PCR) was performed. Both the E and N2 genes were
detected in nasopharyngeal swab. Child was then transferred to the
neonatal intensive care unit (NICU).
On arrival to the NICU, the patient was persistently tachycardic
with heart rates ranging from 190 to 210 BPM and periodically
increasing to 240 BPM. He was successfully treated with adenosine
and infusion of MgSO4 which improved the patient’s heart rate to
170-190 BPM over the next 2 hours. Total parenteral nutrition
was introduced, and mechanical ventilation (SIMV) was continued.
Antibiotics (ampicillin and gentamycin) were also administered
after drawing blood culture. At the opportunity of re-intubation,
the bronchoalveolar lavage fluid was collected for RT-PCR and the
results for E and N2 genes SARS-CoV-2 were positive. It confirmed
diagnosis of COVID-19 in newborn.
From the admission to the NICU, infant was continuously
unconscious and intermittently presented with irritability,
hypotonia or hypertonia and opisthotonos. Routine blood tests
(including glucose, C-reactive protein, procalcitonin, D-Dimer,
ferritin, troponin I, NT-proBNP, and liver enzymes concentrations)
were repeated and blood for culture was obtained (for enteroviruses
and fungi). The laboratory findings showed significantly elevated
blood glucose (504mg/dL), troponin I (682.3ng/mL–normal
value <47.3) and NT-proBNP (52814pg/mL - normal value <125)
concentrations with mildly elevated procalcitonin. At the same
time, C-reactive protein, D-Dimer, ferritin, and liver enzymes levels
were normal. The patient received continuous infusion of insulin
with the increasing doses of 0.05 IU/kg/h to 0.3 IU/kg/h, however
the results of blood glucose measurements were still unsatisfactory
and presented the levels between 300 and 400 mg/dL. Blood
culture was negative either for bacteria or fungi and enteroviruses.
Unfortunately, the blood test with RT-PCR for SARS-CoV-2 genes
was not available at hospital analytical laboratory.
Cerebral ultrasound examination revealed normal size of
ventricles. However, the pictures of thalamus, frontal, and temporal
lobes as well as cerebellum showed hyperechogenic lesions, that
resembled injuries caused by inflammatory or/and ischemic
trauma. The lumbar puncture was performed, and cerebrospinal
fluid was analyzed with respect to bacteria, fungi, enteroviruses,
glycorrhachia, leukocytes count and proteins concentration. A
significantly increased number of leukocytes (2000/mm3) and
raised proteins (967mg/dL) and glucose concentrations (132mg/
dL) were found. Cerebrospinal fluid culture was negative for
bacteria, fungi, and enteroviruses. However, the cerebrospinal fluid
was not tested with RT-PCR for SARS-CoV-2 genes. Despite negative
results of the blood and cerebrospinal cultures, an infusion of
wide spectrum antibiotic (meropenem) was introduced instead
of ampicillin and gentamicin. In addition to antibiotics, the infant
was also treated with dopamine, corotrope, dexamethasone,
pentoxifylline, insulin and diuretics.
Throughout the second day after admission to the NICU,
the parameters of mechanical ventilation were reduced, and
the inspired oxygen fraction lowered to 0.25. The pulmonary
ultrasounds were also normal. Subsequent echocardiography
showed slight improvement in ejection fraction (EF increased from
35% to 45%). The mean arterial blood pressure reached the value
of 53mmHg. However, diastolic disfunction of the left ventricle and
a markedly increased echogenicity of the vascular wall of coronary
arteries were alarming symptoms. Furthermore, the infant was
deeply unconscious, and the series of convulsions appeared
with the escalating frequency. Consecutive cerebral ultrasound
examinations revealed a significantly increasing brain tissue
oedema with extremely reduced blood perfusion, probably because
of markedly elevated intracranial pressure. At the beginning of
the third day after admission to the NICU, a sudden cardiac arrest
occurred. Reanimation was applied promptly and after 3 minutes
the cardiac function was restored. However, within the next 20
minutes the other two episodes of cardiac arrest happened and the
effects of reanimation ended in therapeutic failure. As a reason of
death, the brain herniation was clinically suspected.
On the ground of additional tests performed intravitally, the
congenital inborn errors of metabolism were excluded. The genetic
analysis of DNA microarray also did not show defects, that could
explain clinical symptoms of diabetes mellitus observed in newborn.
The whole exome sequencing (WES) revealed the presence of
known pathogenic mutation c.271dupA (p. Cys91fs. rs549625604)
in one allele of BBS10, which is known to be correlated with
the Bardet-Biedl syndrome (BBS). It is an autosomal recessive
multisystemic human genetic disorder characterized by six major
defects including obesity, mental retardation, retinal degeneration,
renal anomalies, and hypogonadism [1]. In several cases of BBS few
other features such as metabolic defects, cardiovascular anomalies,
hearing loss, hepatic defects and the incidence of diabetes mellitus
have been reported as well. However, the heterozygous carriers of
pathogenic BBS10 mutation are asymptomatic. No other potentially
pathogenic variant was detected by the WES combined with the
copy number variation (CNV) analysis.
The results of autopsy
At the beginning of autopsy, the presence of SARS-CoV-2 RNA in cerebrospinal fluid was tested and real-time polymerase chain reaction (RT-PCR) was performed. Both the E and N2 genes were detected. The results confirmed the wide-spread infection of SARS-CoV-2 in the brain tissue. Macroscopically, the brain tissue had a reduced consistency, its surface was smooth, the sulci were tightened, and the gyri were widened and flat. Histological specimens taken from various parts of the brain revealed the presence of inflammation of small meningeal and cerebral vessels (Figure 1) with endothelial cells apoptosis and multiple microthrombi (Figure 2). Small calcifications in the walls of intracerebral vessels were also observed. The entire picture of the brain corresponded to severe cerebral oedema during acute necrotizing encephalopathy.
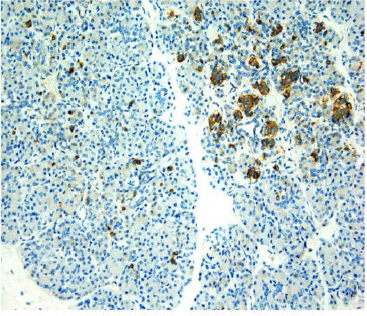
The macroscopic image of the pancreas was normal. Histological specimens stained with hematoxylin-eosin (HE) did not reveal any significant changes or abnormalities (Figure 3) but immunohistochemical reaction with neuroendocrine antibodies (both: chromogranin and synaptophysin) showed extensive damage of the pancreatic islets with significant atrophy of neuroendocrine cells in many areas of the pancreas (Figure 4). The remaining internal organs were properly developed and correctly positioned, with no visible pathologies. Severe brain damage was a direct cause of the infant’s death.
Figure 1: The presence of inflammation of small meningeal and cerebral vessels: hematoxylin-eosin, 40x.
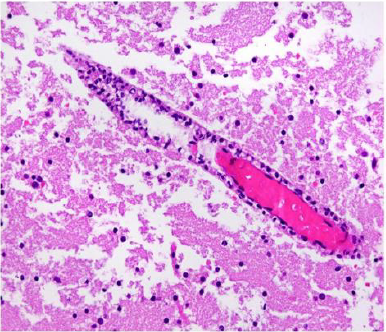
Figure 2: The endothelial cells apoptosis and multiple microthrombi: hematoxylin-eosin, 40x.
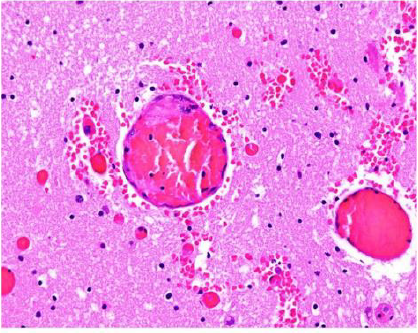
Figure 3: The histological specimens stained with hematoxylin-eosin (HE) did not reveal any significant changes abnormalities: 10x
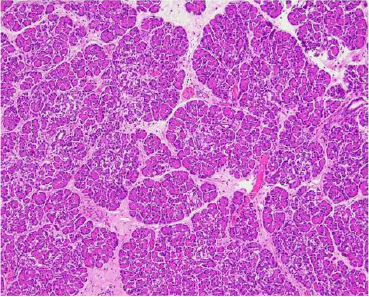
Figure 4: Immunohistochemical reaction with neuroendocrine antibodies (both: chromogranin and synaptophysin) showed extensive damage of the pancreatic islets with significant atrophy of neuroendocrine cells in many areas of the pancreas: 20x.
Discussion
The most frequently encountered presentation of COVID-19
during the neonatal period is asymptomatic or mild infection and
the seemingly uncomplicated course of most cases. Our patient
illustrates fatal disease course of COVID-19 in the newborn.
Except for the hyperglycemia, there were no other neonatal
disorders and important risk factors (e.g., bacterial, enteroviruses
or fungal infections) that potentially might cause these severe
clinical manifestations. Significantly increased inflammatory
response, caused by SARS-CoV-2 infection, that was escalated by
severe hyperglycemia, seem to be the main component of disease
mechanism responsible for clinical and histological findings.
How SARS-CoV-2 affects the brain is not fully understood.
A possible mechanism of entry of the virus into brain is axonal
transport via the cribriform plate, adjacent to the olfactory
bulb [2,3]. The slow circulation in the brain capillaries can also
facilitate the interaction of the viral S protein with the ACE2 on
brain endothelial cells. Moreover, ACE2 receptors have been also
discovered in substantia nigra, ventricles, middle temporal gyrus,
the posterior cingulate cortex, and the olfactory bulb [4].
The damaging effects of neonatal hyperglycemia on the brain
during central nervous system development were reported in
several studies and occurred even without concurrent bacterial
or viral infections [5,6]. The diabetic brain exhibits neurological
alterations in structure, neurotransmitters, electrophysiology,
cognitive function neural density and apoptotic activity [7]. Besides
the induction of pro-apoptotic proteins expression, hyperglycemia
was shown to induce neuronal cell death also due to extracellular
ROS generation which promoted oxidative stress and brain damage
[8]. Also, it is known that hyperglycemia has a significant impact
on the interactions between leukocytes and endothelial cells [9]. It
was found that hyperglycemia increases the rolling, adhesion and
transmigration of neutrophils and macrophages what markedly
stimulates them to produce pro-inflammatory cytokines and
proteolytic enzymes (elastase) [10].
The “inflammatory storm” developing during the SARSCoV-
2 infection causes a significant injury of the endothelial
cells and activates different processes like thrombosis, necrosis,
apoptosis and efferocytosis [11]. Both, thrombosis that occurs
in microcirculation and necrosis that destroys endothelial cells,
devastate the blood perfusion in different organs, including brain
[12]. These mechanisms cause a wide-spread injuries of central
nervous system (Figure 1-2). SARS-CoV-2 was shown to bind to
ACE-2 receptors through its spike proteins. ACE-2 is expressed
in multiple organs, including exocrine and endocrine tissues of
pancreas. Cases of pancreatitis in patients with COVID-19 have been
reported in both adults and children [13]. A study of patients with
diabetes strongly suggests that the localization of ACE-2 expression
in endocrine part of the pancreas allows SARS coronavirus to enter
and damage pancreatic islets, causing acute diabetes [14]. SARSCoV-
2 have been reported to trigger transient insulin resistance
and hyperglycemia.
We found the regular structure of pancreas in the tissue
samples, obtained during autopsy, and stained with hematoxylineosin,
however in samples assessed by immunohistochemistry,
the number of pancreatic islets was significantly decreased
(Figure 3,4). These microscopic images might result from SARSCoV-
2 infection and pancreas damages and may explain why a
severe hyperglycemia was observed in our patients from the
very beginning of the disease. The extremely high blood glucose
concentrations were found even despite the continuous insulin
infusion, what indicated the resistance to insulin and suggested the
extensive damage of pancreas.
Initially, we suspected that the reason for hyperglycemia was
the congenital diabetes mellitus in newborn and as an underlying
comorbidity, it severely complicated clinical course of COVID-19
in infant. However, the results of the genetic analysis did not
show defects, that could explain clinical symptoms of congenital
diabetes mellitus in our patient. Among the wide spectrum of
symptoms of Bardet-Biedl syndrome is diabetes mellitus, however
the symptoms of disease do not appear in heterozygous carriers
of pathogenic BBS10 mutation. Finally, we concluded that SARSCoV-
2 infection, most likely transmitted from the mother to the
newborn, was the primary cause of pancreas damage, and the
main reason for persistent hyperglycemia. On the ground of several
mechanisms that we described above, the extremely high blood
glucose concentrations significantly potentiated and escalated the
inflammatory response, particularly in vascular bed of the brain
and caused the critical oedema, leading to the brain herniation and
death.
In summary, our intent with this case report was to caution
neonatologists and pediatricians against hyperglycemia, caused by
a different reason, that may stimulate the inflammatory reactions
and aggravate the results of treatment of COVID-19 in full-term
newborns.
Ethics statement
The patient’s parents provided informed consent for the publication of this case report.
Author contributions
All authors have made a substantial and intellectual contribution to the work and approved it for publication.s
References
- Stoetzel C, Laurier V, Davis E, Muller E, Rix J, et al. (2006) BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nature Genet 38(5): 521-524.
- Politi LS, Salsano E, Grimaldi M (2020) Magnetic resonance imaging alteration of thew brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 77(8): 1028-1029.
- Le Guennec L, Devianne J, Jalin L, Cao AS, Galanaud D, et al. (2020) Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia 61(8): e90-e94.
- Dahm T, Rudolph H, Schwerk C, Schroten H, Tenenbaum T (2016) Neuro-invasion and inflammation in viral central nervous system infections. Mediat Inflamm 2016: 8562805.
- Rosa AP, Mescka CP, Catarino FM, Luz de Castro A, Teixeira RB, et al. (2018) Neonatal hyperglycemia induces cell death in the rat brain. Metabol Brain Dis 33: 333-342.
- Yang CM, Lin CC, Hsieh HL (2017) High glucose-derived oxidative stress-dependent Heme Oxygenase-1 expression from astrocytes contributes to the neuronal apoptosis. Mol Neurobiol 54(1): 470-483.
- Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY (2000) High glucose-induced apoptosis in human endothelial cells is mediated by sequential activation of c-Jun HN(2)-terminal kinase and caspase-3. Circulation 101(22): 2618-2624.
- Rosa AP, Jacques CE, De Souza LO, Bitencourt F, Mazzola PN, et al. (2015) Neonatal hyperglycemia induces oxidative stress in the rat brain: the role of pentose phosphate pathway enzymes and NADPH oxidase. Moll Cell Biochem 403(1-2): 159-167.
- Booth G, Stalker TJ, Lefer AM, Scalia R (2001) Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab 280(6): E848-E856.
- De Vries AS, Verbeuren TJ, Van De Voorde J, Lameire NH, Vanhoutte PM (2000) Endothelial dysfunction in diabetes, Br J Pharmacol 130(5): 963-974.
- McGonagle D, O Donnell JS, Sharif K, Emery P, Bridgewood C (2020) Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2(7): e437-445.
- Schulert GS, Grom AA (2015) Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Ann Rev Med 66: 145-159.
- Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, et al. (2020) Prevalence, risk factors, and outcomes of hospitalized patients with COVID-19 presenting as acute pancreatitis. Gastroenterology 159(6): 2226-2228.
- Stevens JP, Brownell JN, Freeman AJ, Bashaw H (2020) Covid-19-Associated multisystem inflammatory syndrome in children presenting as acute pancreatitis. J Pediatr gastroenterol Nutr 71(5): 669-671.
© 2021 Lauterbach R. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






