- Submissions

Full Text
Advancements in Case Studies
Recurrence of Glioblastoma Multiforme (GBM) Treated in a Radiotherapy Institution in Chile
Oñoro Guzmán L1*, Hernandez Alvarez V2, Rojas Aladino AR2, Andres Miranda3 and Henriquez M4
1Radio Oncology of the National Cancer Institute of Santiago de Chile, Chile
2Neurosurgeon at Hospital Barros Luco, Chile
3Neuroradiologist at Hospital Barros Luco, Chile
4Oncologist National Cancer Institute of Santiago de Chile/Barros Luco Hospital, Chile
*Corresponding author: Oñoro Guzmán L, Radio Oncology of the National Cancer Institute of Santiago de Chile, Chile
Submission: October 20, 2020;Published: October 30, 2020

ISSN 2639-0531Volume2 Issue5
Abstract
Clinical case: 30-year-old female patient with Glioblastoma multiforme (GBM) IDH (-) MGMT (-) TERT mut, 1p19q, referred in 2018 to the radio oncology service of the National Cancer Institute of Santiago de Chile to complement treatment after surgery macroscopically complete, under the standard schedule of radio-chemotherapy [1]. During the clinical and imaging follow-up after the primary treatment, two recurrences were evidenced in the left parietal occipital region. This evolution is expected in a tumor that tends to recur in 75 to 80% of cases during follow-up, associated with a poor prognosis. Our patient is treated with systemic therapy, vigilant surgery, and fractionated stereotactic radiosurgery (F-SRS) with excellent tolerance and satisfactory evolution.
Conclusion: This case shows the benefit of multidisciplinary treatments for tumors with high aggressiveness, unfavorable prognosis and that tend to recur like GBM. The evaluation of prognostic factors such as age, Karnofsky (KPS), type of surgery and molecular profile help us to offer additional treatments that prolong global survival and improve quality of life in the event of recurrence, which includes irradiation with fractionated stereotactic radiosurgery. AS a valid rescue option in patients with recurrent or residual malignant gliomas.
Keywords: Brain tumors; Glioma; Fractionated stereotactic radiosurgery; Metilación Del promotor De MGMT; Neuroimaging; Temozolomide
Abbreviations: OMS: Organization Mundial De la Salud; MGMT: Metilación Del Promotor De MGMT; GBM: Glioblastoma Multiforme; VMAT: Arcoterapia Volumétrica Modulada Temozolomide; MRI: Magnetic Resonance Imaging; KPS: Karnofsky; ECOG: Eastern Cooperative Oncology Group
Introduction
Glioblastoma multiforme (GBM) is the most common brain tumor of the nervous system with 40% in relation to other malignant tumors of the central nervous system (CNS). The incidence of the disease is 5 to 10 cases per 100,000 person years, and more than 14,000 new cases are diagnosed in the United States each year [2]. It tends to appear in the fifth to sixth stage of life, although it can appear in all ages, including children; it tends to be 1.6 times more common in men [3] than in women. Primary treatment is based on macroscopically complete surgery followed by adjuvant treatment [1,4] of radio chemotherapy according to the standard scheme described by Stupp [1]; with a radiation dose of 60Gy in 30 fractions associated with the concomitant use of an alkylating agent (temozolomide) showing a survival benefit (Sv) of 14.6 months. The addition of adjuvant TMZ increased overall survival by 16.0% at 3 years [5] and the presence of MGMT promoter methylation was the strongest predictor of benefit from TMZ chemotherapy [6].
However, there are other schemes that have shown benefit in frail patients with karnofsky (KPS) of 50% to 70%) and age older than 60 years, reporting a median Sv of 5.1 months for standard radiotherapy (RT) versus 5.6 months for the shortened course (log-rank test, p=0.57) with less use of corticosteroids after treatment [5], which is why it is considered a valid option in this type of patients. Different prospective and retrospective series have proposed shorter schemes with comparable results [7]. The use of stereotaxic radiosurgery (SRS) in the initial treatment of GBM has been postulated as an option. The RTOG 93-05 protocol [8] with very specific eligibility criteria showed that the median Sv in Group 1 was 14.1 months (95% CI: 11.0-14.9) versus 13.7 months (95% CI: 11.3-15.2) in Group 2 (p = 0.53) therefore the best available evidence does not support the use of SRS or F-SRS for high-grade primary gliomas [9]. Despite great efforts to maintain tumor control, recurrence appears in more than 75% [4] in a time interval of 8 months and it is here that the existing treatment options range from surgery, palliative management, use of Systemic therapies such as chemotherapy or endothelial growth factor inhibitors (Bevacizumab), reirradiation or the use of SRS, fractionated stereotactic radiotherapy F-SRS, although these treatments do not prevent the high mortality of GBM if they change the evolution of this pathology.
The precedence of prognostic factors such as KPS, age [5], type of surgery, and molecular markers such as 1p/19q codeletion, IDH1/IDH2 mutation and the presence of promoter methylation (MGMT) have shown prognostic and therapeutic importance [10,11]. Given the recurrence of GBM, we can consider treatments that improve Sv and provide a good quality of life, among these we have the most complete surgery possible avoiding sequelae and deficits, SRS or FSRS and the use of systemic therapies [11]. A greater understanding of the molecular characteristics of gliomas in recent years has helped to stratify patients according to genomic profile [12], seeking a longer survival with good functional capacity.
Clinical Case Presentation
We present the clinical case of a 30-year-old female patient with a progressive headache associated with vomiting and compromised consciousness. At that time the patient was living in Canada, so she was admitted to the emergency service at Foothills Hospital, where she was diagnoses intracranial hypertension secondary to an extensive left occipital tumor with imaging signs of malignancy; macroscopically total resection was performed on April 24, 2018, with post-surgical sequelae of the right hemianopia. He moved to Chile and entered the neurosurgery service at Hospital Barros Luco Trudeau (HBLT) with 100% KPS to continue adjuvant treatment Biopsy (24-April-2018) GBM. WHO (2016) grade: IV DH (-) MGMT (-) TERT mut, 1p19q wildtype. Brain MRI plus spectroscopy (08.08.2018) that rules out tumor remnant or recurrence.
A case is discussed in a multidisciplinary committee where adjuvant treatment with radio chemotherapy under the Stupp scheme (2005) radiation dose of 60Gy in 30 fractions to 2Gy per fractions associated with concomitant temozolomide (75mg/m2) between 10/29/2018 to 12/12/2018 with good tolerance, followed by TMZ for six cycles. Continuous follow-up with magnetic resonance imaging (MRI) of the brain in phase T1/T2 with gadolinium every three months and then every 6 months.
In November 2019, the dreaded recurrence on follow-up with MRI of the brain appeared in the left parieto occipital, for which a second surgery with neuromonitoring was performed, achieving total resection of the tumor in November 20, 2020, the Biopsy is compatible with NOS Glioblastoma, recurrent. HDI negative, p53 negative. Ki67 40%. She received adjuvant systemic therapy with temozolomide 75mg/for 6 cycles with good clinical response. Despite the local and systemic treatments received, signs of progression of a left parieto occipital solid cystic lesion appear, associated with impaired gait, headache, and diplopia. Therefore, a tumor resection was performed via vigilant craniotomy with neuromonitoring on 06.03 2020. A biopsy was taken, and no complications were described. 24 hours after surgery, MRI of the brain was performed, showing a moderate mass effect on the convex furrows and a resection of approximately 90% of the capturing tumor (Figure 1).
Figure 1: Axial MRI section of the brain where we see 1 image of the parieto-occipital cystic solid recurrence, the second image corresponds to the control two months where there is a marked tumor progression with heterogeneous enhancement to gadolinium measuring 8.4 x 4.5 x 4.9 cm. The third image corresponds to the immediate postoperative period where a resection of approximately 90% of the capturing tumor is considered.
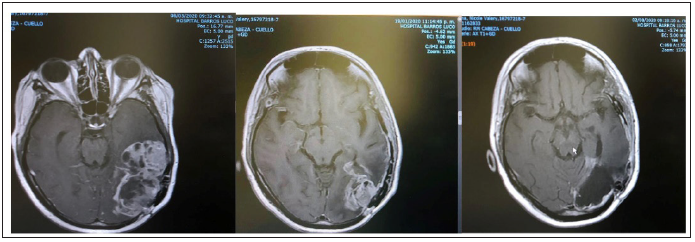
The result of this new recurrence is compatible with NOS Glioblastoma, recurrent. HDI negative, p53 negative. Ki67 40%. Given the aggressiveness of GBM, recurrences are to be expected and this time adjuvant systemic therapy is changed to lomustine 110mg/m2 every six weeks and it is decided to perform an F-SRS treatment on remnant 23mm tumor close to the operative bed as seen in the Figure 2. At a dose of 35g and in 10 fractions (3.5Gy per fraction). Immobilization with frameless thermoplastic mask and neck immobilization with moldable Accu cushion, plus mouth opening plus bite blocking device (Figure 3).
Figure 2: Axial section of MRI in phase T1 with gadolinium showing a lesion with intense left basal temporal enhancement.

Figure 3: Patient placed in simulation scanner with immobilization attachments for radiosurgery.

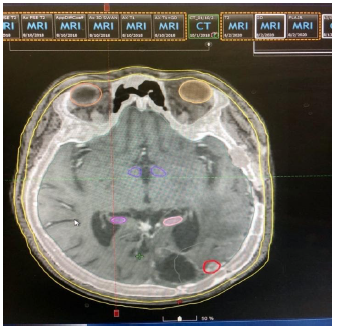
Dosimetry is performed in an eclipse planner, with VMAT technique in linear accelerator (LINAC) with 6x energy. The definition of organs at risk (OARs) was based on the references of the atlas European practical Therapy Network (EPTN) [11] (Figure 4). The target volume for planning was determined when it was considered equivalent to the GTV (palpable or visible tumor volume) established as the tumor remnant without inclusion of the edema plus a CTV () that is equivalent to the GTV without enlargements. followed by PTV is the CTV plus margin 3mm Tumors were treated with the 90% isodose line (Figure 5).
Figure 4: www.cancerdata.org

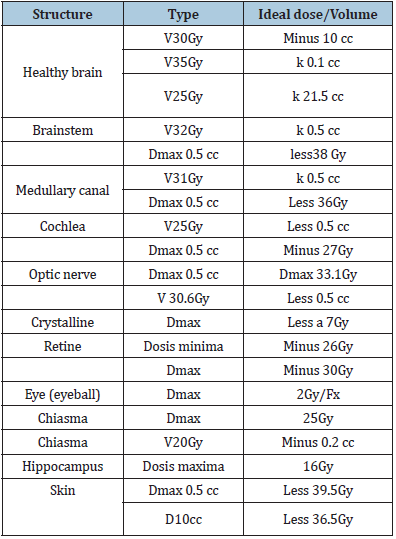
Figure 5: The delimitation of risk organs is observed and in red we can see the tumor CTV plan evaluation we use the dosimetry from the above plan as a reference only. The parameters established for restriction in tumor and organs at risk are described in Table 1.
Patient completes treatment for tumor recurrence with H-SRS on September 22, 2020 with excellent tolerance and low doses of corticosteroids (4mg of dexamethasone every day) a 90% KPS and ECOG 1; Therefore, the benefit of H-SRS in the treatment of relapses or remnants due to GBM is supported [13,14]. Treatment monitoring is based on brain MRI and monthly clinical evaluations.
Table 1:
Discussion
We consider that patients with the most aggressive adult brain tumor, with an infiltrative nature and an evaluation towards local recurrence of 75 to 80% [1,2], which has a mean survival of 12-18 months with treatment standard [1,4] and that has been translated for decades as a challenge for neurosurgeons and radiation oncologists, who in the face of efforts to prolong tumor control have studied the benefits of primary treatments and treatments for the dreaded recurrence. The existence of molecular factors such as the presence of methylguanine-DNA-methyltransferase (MGMT), which is considered a predictive marker for a longer survival with the use of alkylating agents [10] and a better response to the management of relapse, therefore the knowledge and application of genomics in the GBM is increasingly applicable.
The functional status, age, symptoms in the event of recurrence, the possibility and type of surgery allow us to establish patients eligible for additional treatments in the event of recurrence and to estimate the clinical results; Surgical resection is often the first option to alleviate symptoms, followed by rescue systemic therapy or re-irradiation [10], bearing in mind that only a select number of patients are healthy enough to withstand surgery [7]. Systemic therapies offer a modest 8-month survival benefit from recurrence. The use of TMZ has proven to be an effective rescue therapy [8] although the greatest contribution of this alkylating agent has been as a radiosensitizer [8,9] and nitrosoureas.
In a phase 3 trial for recurrent GBM, no survival benefit was seen with the addition of bevacizumab to lomustine compared to lomustine alone. Bevacizumab (BEV) that has emerged as an effective systemic treatment for recurrent GBM, replacing TMZ as the standard of care [6,11,15-18]; Given the high cost, its access is difficult in Latin American countries, so its use and maintenance is not widespread and it is a treatment not free of serious adverse effects such as hypertension, thromboembolism and ventricular failure among others [15]. The application of a minimally invasive treatment, with high conformation and that allows to give high doses per fraction such as SRS/F-SRS have the power to improve the therapeutic relationship by administering high biologically effective doses while reducing high doses to normal brain tissues. Knowing that local recurrence represents an important challenge in the treatment of patients with GBM, since the nineties series have been published that have shown survival of 5.7 to 14.3 months [3,10,12] (median of 10 months) with doses between 30 at 37 Gy [3,12] with a risk of radio-necrosis of 8%.
In our clinical case, we applied the H-SRS technique with a dose of 35Gy in 10 fractions used in the retrospective series with excellent tolerance and a mean Sv of 11 to 8 months after H-SRT regardless of reoperation or surgery concomitant chemotherapy [15] The median survival time (MST) from the date of diagnosis (based on Kaplan-Meier estimates) was 23 months (95% CI, 18 to 26 months). In multivariate analysis, factors influencing overall survival were younger age at diagnosis (p 0.001), smaller and smaller GTV (p 0.001). We note that patients who experienced a recurrence within 6 months of initial treatment had an unexpectedly good prognosis, suggesting that they should not be disqualified from H-SRT or other salvage therapy.
At present, irradiations are supported by the use of neuroimaging, including spectroscopy, which allows describing the metabolism of tissues and distinguishing progression radio-necrosis. Imaging technique widely used in tumors where surgical resection or biopsy is very complex; as in patients with brain stem gliomas [12]. Knowing that the treatments before the recurrence of GBM in the present do not remove the mortality generated by this tumor that increasingly appears in young people and with a good KPS. We must continue to offer our patients treatment options that prolong life and quality of life, which is why the H-SRS/SRS are a valid treatment option in high-grade gliomas [11].
Treatment in cases of recurrence is not curative, there are no randomized clinical studies that compare surgery, systemic treatments and radiation with each other. Therefore, patients with recurrent GBM do not have a standard treatment, they could be treated with surgery, surgery plus SRS/FSRS or systematic treatments, the decision to follow treatment should be based on the clinical characteristics of the patient, functional status, KPS, tumor location and access to treatment by health services. These results warrant a prospective evaluation of H-SRT in future studies as standard salvage therapy for previously irradiated patients.
Current efforts are focused on the development of new treatment approaches, including molecular targeted agents and immunotherapies. our understanding of the disease is improving the analysis of useful prognostic and therapeutic factors to choose the best treatment option or combination among those that already exist.
Conclusion
Fractional stereotactic RT appears as a beneficial treatment option in previously irradiated GBM relapse, since it allows high doses of radiation to be administered in various fractions, minimizing normal tissue toxicity and keeping the patient away from the complications or sequelae of re-surgeries.
References
- Stupp R, Mason WP, Vanden Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10): 987-996.
- Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol 14 (Suppl 5): 1-49.
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, et al. (2014) The epidemiology of glioma in adults: a ‘‘state of the science’’ review. Neuro Oncol 16(7): 896-913.
- Stupp R, Hegi ME, Mason WP, Vanden Bent MJ, Taphoorn MJ, et al. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5): 459-466.
- Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, et al. (2004) Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: A prospective randomized clinical trial. J Clin Oncol 22(9): 1583-1588.
- Fogh SE, Andrews DW, Glass J, Curran W, Glass C, et al. (2010) Hypo-fractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28(18): 3048-3053.
- Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, et al. (2015) International atomic energy agency randomized phase iii study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 33(35): 4145-4150.
- Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, et al. (2000) Single dose radio-surgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47: 291-298.
- Curran WJ, Scott CB, Horton J, Nelson JS, Weinstein AS, et al. (1993) Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 85(9): 704-710.
- Weller M, Stupp R, Hegi ME, Bent MV, Tonn JC, et al. (2012) Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol 14(suppl 4): 100-108.
- Eekers DBP, Int Ven L, Roelofs E, Postma A, Troost EGC (2017) EPTN international neurological contouring atlas. Cancer Data.
- Nayak L, Reardon DA (2017) High-grade Gliomas. Continuum (Minneap Minn). 23(6, Neuro-oncology): 1548-1563.
- Chong Zhou, Youyou Xia, Pei Huang, Luan Guan, Xiaoming Shen, et al. (2020) Fractionated stereotactic radiation therapy using volumetric modulated arc therapy in patients with solitary brain metastases, BioMed Research International 2020:6342057.
- Marcrom SR, McDonald AM, Thompson JW, Popple RA, Riley KO, et al. (2017) Fractionated stereotactic radiation therapy for intact brain metastases. Adv Radiat Oncol 2(4): 564-571.
- Sallabanda K, Yañez L, Sallabanda M, Santos M, Calvo FA, et al. (2019) Stereotactic radiosurgery for the treatment of recurrent high-grade gliomas: Long-term follow-up. Cureus 11(12): e6527.
- Niranjan A, Kano H, Monaco EA, Lunsford LD (2019) Salvage Leksell stereotactic radiosurgery for malignant gliomas. Prog Neurol Surg 34: 191-199.
- Roberge D, Souhami L (2003) Stereotactic radiosurgery in the management of intracranial gliomas. Technol Cancer Res Treat 2(2): 117-125.
- Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, et al. (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343(19): 1350-1354.
© 2020 Oñoro Guzmán L. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






