- Submissions

Full Text
Advancements in Case Studies
Relation Between the Account of Neutrophils to Exit and Risk of Hospital Re-Entry in Pediatric Patients with Fever and Neutropenia with Acute Lymphoblastic Leukemia
Almaguer-Garza E*, Jiménez-Antolinez V, Castillo-Bejarano JI, Gómez-Alcorta R, Vaquera-Aparicio DN, De la O-Cavazos ME and Mascareñas-de los Santos AH
Department of Pediatrics, Mexico
*Corresponding author: Estefanía Almaguer Garza, Department of Pediatrics, University Hospital “Dr. José Eleuterio González” Universidad Autonoma de Nuevo Leon (U.A.N.L.), Mexico
Submission: April 16, 2020;Published: May 05, 2020

ISSN 2639-0531Volume2 Issue4
Abstract
Introduction: Fever and neutropenia (FN) are common complications in children receiving chemotherapy for cancer. Although various guidelines for the management of fever and neutropenia have been developed, none are dedicated to children.
Materials and methods: Observational, longitudinal, descriptive, non-comparative and retrospective cohort. The study was conducted in a total of 88 pediatric patients with a diagnosis confirmed by the Hematology Service of acute lymphoblastic leukemia who have been admitted due to fever and neutropenia for the administration of intravenous antibiotics during the period from 2016 to 2018.
Results: 88 patients were included, mostly 52% male, with a mean age of 5.8 years ±7, an average hospital stay of 5.7 days ±2.37, a total leukocyte count upon admission of 125 cells and discharge of 592 cells and that in 82% of cases it was not possible to establish an etiologic causal agent.
Conclusions: The numbers of leukocytes at discharge between 100-199 cells per field were not significantly associated with a hospital readmission during the analysis, instead the figures below 100 neutrophils were significantly associated (p: 01), therefore that we consider a sum between 100-199 cells to be safe.
Keywords: Acute lymphoblastic leukemia; Fever; Neutropenia; Pediatrics; Neutrophils; Hospital readmission
Introduction
Fever and neutropenia in cancer patients have been considered medical emergencies for the past decade [1]. They require prompt evaluation and hospital administration of broad-spectrum intravenous antibiotics, the aggressive approach has been universally recommended because approximately 60% of febrile neutron episodes are caused by bacterial infections (with or without bacteremia), a condition that was difficult to identify in many children [2,3]. In two consecutive studies, it was concluded that serial determination of serum C-reactive protein can help in the identification of children at high risk of bacterial infection and for selected children at low risk of bacteremia, antibiotic treatment may be stopped safe on the third day of hospitalization [4]. Fever and neutropenia (FN) are common complications in children receiving chemotherapy for cancer. Although various guidelines for the management of fever and neutropenia have been developed, none are dedicated to children [5,6]. Fever was defined as an axillary temperature of 38.5 °C or higher in one measurement or 38 °C or more in two consecutive measurements separated by 1-hour intervals [2]. Severe neutropenia was defined as a neutrophil count less than or equal to 500/mm3. Use of monotherapy with a beta-lactam anti-pseudomone or a carbapenem as empirical therapy in pediatric patients at high risk of FN [2]. In one study, a neutrophil count below 100/mm3 and a temperature of 39 °C or higher were significantly associated with an increased risk of bacteremia [7].
In 2000, the Multinational Association for Cancer Supportive Care (MASCC) published a risk index for FN, identifying low-risk patients potentially suitable for outpatient therapy. It is based on the clinical characteristics obtained in the presentation of the patient, without laboratory results. A score of ≥21 was recommended as the low-risk threshold [8,9]. Early discharge with outpatient management may be possible for low-risk patients (class I), short-term observation to monitor the development of adverse effect events could safely manage intermediate-risk patients (class II), and admission of hospitalized patients must be justified for high-risk patients (class III) [10]. Children admitted with fever and neutropenia could be discharged after a minimum of 48 hours without additional antibiotic treatment once they had been afebrile for 24 hours with negative blood cultures since initial presentation, regardless of their neutrophil count [11]. We conducted a retrospective. Review regarding readmissions and subsequent documented documents. Infections in patients with fever and neutropenia discharged with a neutrophil count of less than 500 cells/mm3. Discharge in patients with persistent neutropenia who are afebrile with negative blood cultures produces acceptable rates of readmission and subsequent infection and does not lead to increased morbidity and mortality [4,12]. The hypothesis proposed was that the number of neutrophils at discharge in febrile neutropenic patients diagnosed with Lymphoblastic Leukemia represents a significant prognostic factor for hospital readmission. The objective of this study is to determine the relationship between the neutrophil count at discharge and the risk of hospital readmission in pediatric patients with fever and neutropenia with acute lymphoblastic leukemia.
Materials and Methods
It is a retrospective, observational, longitudinal, descriptive and non-comparative study, which was decided to do because the panorama of our institution is apt to be carried out because the University Hospital Dr. ’Dr. José Eleuterio González ’’ from the Autonomous University of Nuevo León represents a landmark for hematological patients in Northeast Mexico with a very high influx of patients; who have been diagnosed with acute lymphoblastic leukemia or other hematological malignancies and therefore an investigation of this type can be carried out to obtain results that could be extrapolated to daily clinical practice for the benefit of our patients.
The inclusion criteria were: Pediatric patients (up to 15 years 11 months and 28 days), patients with a diagnosis of acute lymphoblastic leukemia made by the Pediatric Hematology Service of this hospital, reason for hospital admission: fever under study (higher body temperature at 38 degrees Celsius) and neutropenia (less than 1500/μL in blood cell biometry at admission), complete and readable clinical record, with blood and urine cultures recorded in the record and in the bacteriology database, with two serial blood cell biometries prior to discharge from hospital, C-Reactive Protein registry at admission and discharge. The following exclusion criteria were taken into account: Hospital stay of less than 24 hours or transfer to another center after their initial admission, non-pediatric patients, patients undergoing post-transplant bone marrow treatment, patients with other types of leukemias other than ALL , patients without fever or neutropenia on admission, patients who did not have blood or urine cultures, lack of recording of blood biometries on admission or discharge for any reason, failure to register PCR on admission or discharge for any reason. The elimination criteria were: Patients with non-hematological malignancies, incomplete clinical record and patient death.
An analysis of the database of the Hematology Service (with written authorization from the head of the service and participation of a doctor member of the Hematology Service) was performed to select those patients who met the inclusion criteria and we searched for those who have been admitted for fever and neutropenia for the administration of intravenous antibiotics during the period from 2016 to 2018 and variables were registered in the same way, a sub-database was created with the information collected and filtered with the clinical record and with the service database From Hematology (as well as from other intrahospital sources such as the Bacteriology, Imaging and General Laboratory services), it was not necessary at any time to identify the patient for the purposes of this study, only working with their registration number on a database private data accessible only to research team on a single computer , later on these obtained data a statistical analysis was carried out which will be described later.
The variables of interest were: age, date of admission and discharge, number of days admitted, values of Blood Biometry at admission and discharge (hemoglobin and platelets), neutrophil values at admission and discharge, CRP values at admission and at discharge. discharge, blood culture results, uro cultures or other cultures (CSF, soft tissues, feces, tracheal secretion) (microbiological isolation), changes in the chest radiograph, phase of treatment in which it is found, type of chemotherapy received, use of prophylaxis antimicrobial used, time relationship between start of chemotherapy and onset of fever, number of days with an absolute neutrophil count less than 100/𝜇l, number of days with an absolute neutrophil count less than 500/𝜇l. type of antibiotic or antifungal and number of days with each, duration of fever, stay in PICU, clinical focus of the infection (if known), comorbidities (mucositis, pancreatitis, pneumonia, etc.), complications (hypotension , sepsis, etc.), need to transfuse blood products and mortality.
This protocol was sent for authorization to the Ethics Committee and Research Committee of the University Hospital "Dr. José Eleuterio González” of the U.A.N.L. and was authorized with the registration number PE19-00020. This protocol does not provide any type of financial or commercial gain for its realization. It was performed using the SPSS version 21.0 computer program for Mac®. Descriptive statistics. Mean, standard deviation, 95% CI, minimum result and maximum result were obtained for each measurement parameter included in the present study. Inferential statistics. Two-tailed parametric correlation tests (ANOVA and t-student) were performed to determine if there are significant differences between the average results obtained between the different groups for each measurement parameter, in the same way two-tailed t-student tests were performed to determine if there are significant differences between the means of each parametric measurement parameter between the different study groups, taking as significant a value of p less than 0.05. The chi-square test was used for the categorical variables. Similarly, a univariate and multivariable regression analysis was performed to detect association patterns between specific variables (especially the neutrophil count at discharge and the risk of hospital readmission).
Results
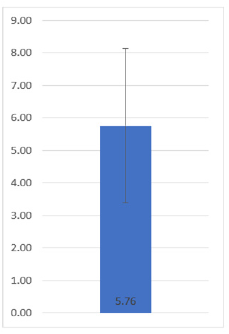
A total of 88 patients were included in the present study (in accordance with what was obtained in the calculation of the sample size), who fulfilled the inclusion criteria previously mentioned. Of all the patients, 52 belonged to the male gender (59.09%) and 36 to the female gender (40.91%), finding no significant differences regarding gender (p: 0.12). Of the 88 pediatric patients who were diagnosed with leukemia, it was reported that together they had an average age of 5.8 years ± 3.7 (Figure 1). Of all the patients, 30 were from the Monterrey metropolitan area (34.09%), 22 were from other municipalities in the state of Nuevo León outside the Metropolitan Area (25%), and 36 were from other states (40.91%). The days that the patients remained hospitalized until discharge were quantified, thus being an average result of 5.76 ± 2.37 days (Figure 2).
Figure 1: Average age and standard deviation of the patients included in the study.
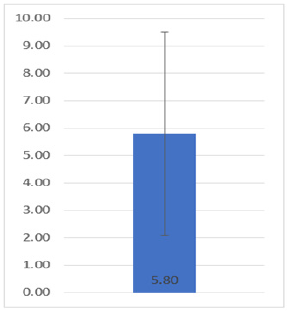
Figure 2: Average and standard deviation of the length of hospital stay of the patients included in the present study.
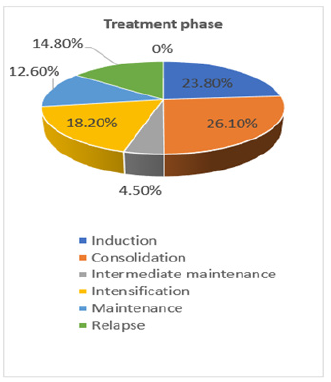
Neutrophil counts have been carried out on all patients both when patients are admitted to the hospital, during their stay and when they are discharged, thus collecting the number of neutrophils that patients had during the high being this amount of 125 ± 145.64 (range 1-500), note that the standard deviation is higher than the mean which tells us about the great variation between the parameter results. Regarding the number of neutrophils at discharge, it was observed that the number of neutrophils increased significantly the amount at the time of discharge with an average amount of 592.74 ± 957.89 (range 3-3640), note that in this case the standard deviation is also above average (Figure 3). All the patients were in different stages of treatment, where 23 (26.1%) were in the consolidation stage, 21 (23.8%) in induction, another 16 (18.2%) in the intensification stage, 13 (14.8%) patients relapsed 11 (12.6%) were in the maintenance stage as well as 4 (4.5%) in the intermediate maintenance stage and not a single patient (0%) was reported in the refractory stage (Figure 4).
Figure 3: Amount of neutrophils on admission and discharge of the patients included in the present study.
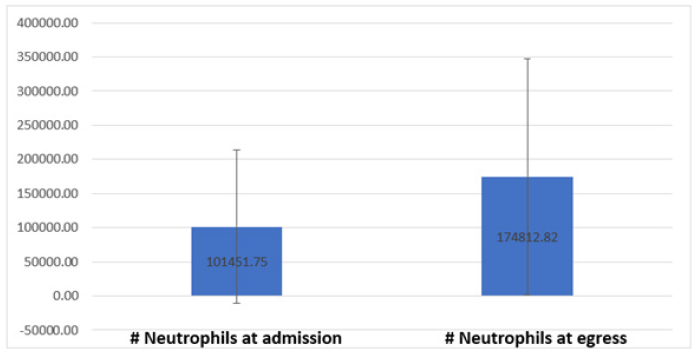
Figure 4: Distribution of the treatment stages in which the different patients included in the study were.
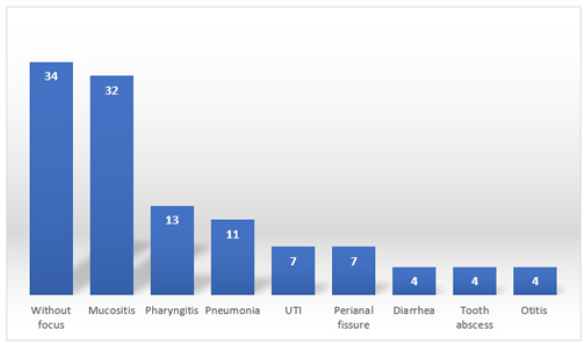
The 88 patients in the study presented various comorbidities, some of them had more than 1, in this way a count was made of the number of patients who arrived, and it was reported that 34 (38.6%) were reported without a focus, 32 ( 36.3%) presented mucositis, 13 (14.7%) pharyngitis, 11 (12.5%) patients reported pneumonia, 7 (7.9%) showed ICU, another 7 (7.9%) reported a perianal fissure, 4 (4.5%) showed diarrhea , another 4 (4.5%) with a dental abscess and 4 (4.5%) more with otitis (Figure 5). The average of the days that passed from the last chemotherapy to the fever and neutropenia symptoms was 9.76 days ± 7.12. The number of monocytes reported by patients on admission, as well as on discharge, was compared, while the number reported at the time of entry was an average of 333.41 ± 423.83 as well as the average increase at the time of exit with an average number of 433.77 ± 377.43. Hemoglobin values at admission and discharge were quantified, obtaining an average result of 8.95mg/dl ± 2.00 at admission and increase to an average number of 10.73mg/dl ± 1.55 at discharge. The platelet value reported by patients at admission was 101451.75 ± 112599, increasing their number to 174812.82 ± 172959.58 at discharge. Patients reported an average CRP level of 8.45mg/dl ± 4.90 at the time of admission. Throughout the investigation, it was reported that patients presented fever for 2 ± 1.4 days on average, after which it subsided. The urine culture performed on the patients in 64 (72.7%) of the cases reported negative and positive in 24 (27.3%) of the cases. Peripheral blood cultures performed on patients in 83 (94.3%) of the cases reported negative, as well as 5 (5.7%) of the cases reported positive.
Figure 5: Absolute frequencies of the comorbidities presented by the patients included in the present study.
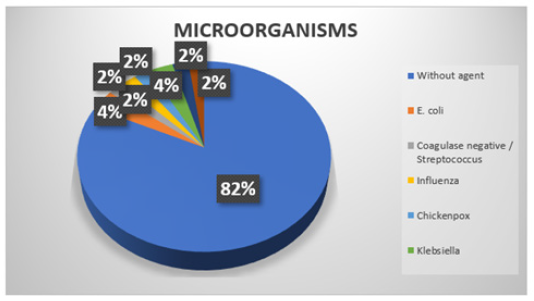
At the time of investigating which microorganisms were present in the cultures of the patients, it was discovered that in 72 (81.8%) of the cases no agent was shown, in 3 (3.4%) of the patients it showed an infection by E. Coli, as well as in 3 other (3.4%) patients were positive for Klebsiella, in the rest of the patients coagulase negative/streptococcus, pseudomonas and coagulase negative infections were detected in 2 (2.2%) cases each. It was identified by clinical varicella, influenza by rapid test (Figure 6). Globular packages were transfused in 13 (14.8%) of the patients, while the remaining 75 (85.2%) were not. Platelet concentrates were transfused in 16 (18.2%) of the patients, while the remaining 72 (81.8%) were not transfused with platelet concentrates. Of the 88 patients in total in the study, only 7 (8%) re-entered after discharge, while the other 81 (92%) did not report readmission. The reported causes for which the patients have been readmitted were that 3 (3.4%) reported fever, 2 (2.3%) presented hyporexia, 2 (2.3%) an ecthyma appeared on the skin and in the other 81 (92%) patients does not apply because they did not re-enter. In two patients (2.3%) their death was reported during the study. The percentage of patients in which it was necessary to admit them to PICU was 9 (10.2%).
Figure 6: Distribution of the isolated microorganisms in the present study.
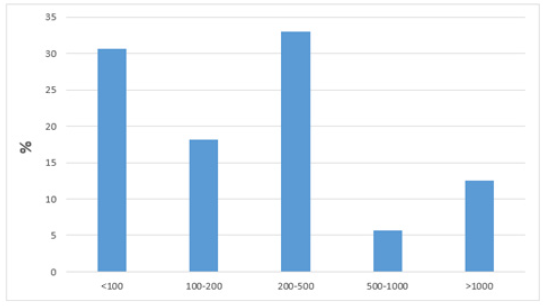
Patients at discharge recorded their neutrophil count where subdivisions were made by groups where patients who reported a neutrophil level <100, 100-200, 200-500, 500-1000 and >1000 are found. In the group that reported a number of neutrophils <100, 27 (30.6%) patients were found, in the group of 100-200, 16 (18.2%) patients were observed, the group of 200-500 contained 29 (33%), this being the most abundant group in terms of patients, in the 500-1000 group 5 (5.7%) patients were found, in the <1000 group 11 (12.5%) patients were observed (Figure 7). Multivariate analysis was performed using logistic regression. For the multivariate analysis, variable cut-off values were selected to divide the patients into equal groups that were clinically significant. In particular, a cutoff value for a CAN of 100 cells/mm3 was chosen as previous reports have identified a lower level as a risk factor for developing significant infection.
Figure 7: Percentage of neutrophils at discharge in subgroups.
Multivariate analysis identified a diagnosis of ALL and a discharge ANC 100 cells/mm3 as significantly associated with readmission during the same neutropenic period. ALL, support ANC 100 cells/mm3 and download. 100 ANC cells/mm3 were found to be risk factors for being diagnosed with significant infection after readmission. Values above 100 were not significant and could be taken as insurance.
Discussion
Specific evidence-based pediatric guidelines are available for the management of FN. These guidelines include high standard criteria such as that the patient must be afebrile for at least 24 hours, blood cultures that must be negative for 48 hours, the patient must have evidence of bone marrow recovery at discharge. CAN is used as a surrogate marker for bone marrow recovery [13,14]. Previous studies have shown that discharged patients with a CNA <500/𝜇l are not at increased risk of adverse infectious outcomes, even in cases of prolonged neutropenia lasting more than 7 days; if they did not meet the standard discharge criteria and have hematological evidence of bone marrow improvement [15]. The previously published guidelines suggest a post-swim CNA>100/𝜇l as a reasonable threshold value for evidence of bone marrow recovery and discharge safety, but there are no studies cited in this recommendation with specifically compared patient outcomes for values. CNA threshold <500/𝜇l [16]. A more recent study showed that patients who were discharged with a CNA of <100/𝜇l had a triple increase in readmission for fever compared to patients with a discharge and CNA >100/𝜇l [13].
However, this study also did not stratify the results for patients with discharge CNA values between 100/𝜇l and 500/𝜇l. Post-discharge readmission rates and fever recurrence were similar among CNAs in the discharge subgroups of 100-199/𝜇l, 200-499/𝜇l and ≥500/𝜇l, and few re-entry infections were detected in the bloodstream in either of the groups. Overall, there is insufficient data to conclude on discharge safety for a CNA <100/𝜇l. However, these data suggest that there are equivalent results for patients discharged with a CNA of 100-199/𝜇l compared to those discharged at a higher threshold to support safe discharge at home without continued antibiotic therapy in those with a CNA >100/𝜇l, supporting published guidelines. Since recovery of the monocyte count may precede recovery of the neutrophil count, some providers consider an increase in CAM as an early indicator of impending marrow recovery when considering discharge from patients admitted for FN [17,18].
In the present study, all primary outcomes, including readmission rate, were higher for episodes with CNA <500/𝜇l compared to those with CNA ≥ 500/𝜇l. It is noteworthy that there were no readings or new episodes for episodes with CNA ≥ 500/𝜇l and CNA <200/𝜇l, but only seven of these episodes had a CNA <100/𝜇l. These results suggest that a CAN> 500/𝜇l in the discharge, even in the case of a CNA <100/𝜇l could be a reasonable strategy. This would need to be evaluated in a larger cohort before conclusions can be drawn. The use of CNA >100/𝜇l as a threshold for home discharge of empirical antibiotics may result in an additional benefit with respect to hospital costs and a lower risk of nosocomial infection [19,20]. Reid et al. [21] mentioned that virus infections may therefore be associated with depression of neutrophil function in a group of children whose neutrophils may already be adversely affected by their leukaemia or by treatment. This depression of function, in addition to the risks of neutropenia, may make children with ALL even more susceptible to bacterial and fungal infections, and shows the seriousness of comparatively common viruses to such children [21].
There are several limitations in this study. The data were obtained through a review of the retrospective table and were based on precise and detailed documentation in the medical record. This was particularly noticeable when the indications for continued hospitalization were determined after meeting the standard discharge criteria. Parameters such as poor oral intake status and the need for IV medications were verified in the table, but this could have been incompletely documented. Episodes were excluded if a bacterial infection from any source of fever was identified and treated with targeted antimicrobial therapy. The sample size is modest and therefore has limited power. The event rate was low for all primary outcomes. Particularly remarkable, very few patients were discharged with a CNA <100µl. This limits the conclusions that can be made on the discharge of patients with an ANC <100𝜇l. In general, the equivalent safety results for patients discharged with a CNA of 100-199𝜇l compared to discharges with a higher threshold support the previous recommendation of a post-nadir CNA >100𝜇l as a safe threshold for discharge from hospital without empirical antibiotics. Furthermore, compliance with this recommendation has the potential to reduce unnecessary utilization of medical care and nosocomial morbidity. Prospective implementation is needed to assess changes in outcomes and utilization of medical care to establish the safety and efficacy of using this threshold and further consolidate the recommendation.
Conclusion
In our cohort of patients, we identified that patients with neutrophil values at discharge above 100𝜇l are safe due to the low rate of readmissions that occurred above this sum, in the same way all cases of readmission presented a count below 100. The predominant characteristics were male gender, childhood, high risk level for ALL, early discharge, infectious focus of respiratory and digestive origin and antibiotic therapy with vancomycin. The predominant laboratory characteristics were isolation of Gram-positive germs and not being able to isolate the causative microorganism.
References
- Santolaya ME, Alvarez AM, Becker A, Cofré J, Enríquez N, et. al. (2001) Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. Journal of Clinical Oncology 19(14): 3415-3421.
- Lehrnbecher T, Phillips R, Alexander S, Alvaro F, Carlesse F, et al. (2017) Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. Journal of Clinical Oncology 30(35): 4427-4438.
- Ahn S, Lee YS, Lee JL, Lim KS, Yoon SC (2016) A new prognostic model for chemotherapy‑induced febrile neutropenia. Int J Clin Oncol 21(1): 46-52.
- Villanueva, August KJ (2016) Early discharge of neutropenic pediatric oncology patients admitted with fever. Pediatric Blood Cancer 63(10): 1829-1833.
- Campbell ME, Friedman DL, Dulek DE, Zhao Z, Huang Y, et al. (2017) Safety of discharge for children with cancer and febrile neutropenia off antibiotics using absolute neutrophil count threshold values as a surrogate marker for adequate bone marrow recovery. Pediatric Blood Cancer 65(3).
- Loeffen EA, Te Poele EM, Tissing WJ, Boezen HM, De Bont ES (2016) Very early discharge versus early discharge versus non-early discharge in children with cancer and febrile neutropenia. Cochrane Database Syst Rev.
- Griffin TC, Buchanan GR (1992) Hematologic predictors of bone marrow recovery in neutropenic patients hospitalized for fever: implications for discontinuation of antibiotics and early discharge from the hospital. J Pediatr 121(1): 28-33.
- Aquino VM, Buchanan GR, Tkaczewski I, Mustafa MM (1997) Safety of early hospital discharge of selected febrile children and adolescents with cancer with prolonged neutropenia. Med Pediatr Oncol 28(3): 191-195.
- Aquino VM, Tkaczewski I, Buchanan GR (1997) Early discharge of low-risk febrile neutropenic children and adolescents with cancer. Clin Infect Dis 25(1): 74-78.
- Cohen KJ, Leamer K, Odom L, Greffe B, Stork L (1995) Cessation of antibiotics regardless of ANC is safe in children with febrile neutropenia. A preliminary prospective trial. J Pediatr Hematol Oncol 17(4): 325-330.
- Lehrnbecher T, Stanescu A, Kuhl J (2002) Short courses of intravenous empirical antibiotic treatment in selected febrile neutropenic children with cancer. Infection 30(1): 17-21.
- Hodgson-Viden H, Grundy PE, Robinson JL (2005) Early discontinuation of intravenous antimicrobial therapy in pediatric oncology patients with febrile neutropenia. BMC Pediatr 5(1): 10.
- Rondinelli PI, Ribeiro KC, DeCamargo B (2006) A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. J Pediatr Hematol Oncol 28(10): 665-670.
- Allen RC, Holdsworth MT, Johnson CA, Chavez CM, Heideman RL, et al. (2008) Risk determinants for catheter-associated blood stream infections in children and young adults with cancer. Pediatr Blood Cancer 51(1): 53-58.
- Ammann RA, Bodmer N, Hirt A, , Niggli FK, Nadal D, et al. (2010) Predicting adverse events in children with fever and chemotherapy-induced neutropenia: The prospective multicenter SPOG 2003 FN study. J Clin Oncol 28(12): 2008-2014.
- Baorto EP, Aquino VM, Mullen CA, Buchanan GR, DeBaun MR (2001) Clinical parameters associated with low bacteremia risk in 1100 pediatric oncology patients with fever and neutropenia. Cancer 92(4): 909-913.
- Klaassen RJ, Goodman TR, Pham B, Doyle JJ (2000) “Low-risk” prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol 18(5): 1012-1019.
- Alexander SW, Wade KC, Hibberd PL, Parsons SK (2002) Evaluation of risk prediction criteria for episodes of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol 24(1): 38-42.
- Lehrnbecher T, Phillips R, Alexander S, Alvaro F, Carlesse F, et al. (2012) Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol 30(35): 4427-4438.
- Rackoff WR, Gonin R, Robinson C, Kreissman SG, Breitfeld PB (1996) Predicting the risk of bacteremia in children with fever and neutropenia. J Clin Oncol 14(3): 919-924.
- Reid MM, Craft AW, Low WT (1979) Neutrophil function in children with acute lymphoblastic leukaemia in the presence and absence of viral infections. Arch Dis Child 54(8): 619-622.
© 2020 Almaguer-Garza E. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






