- Submissions

Full Text
Advancements in Case Studies
CREALYS®: New Product for Man Infertility in-vivo and in-vitro Results
Maggioni C1*, Veit J2, Diaz P2 and Dalmartello M3
1Via Rembrandt, Italy
2Department of Biomedical and Pharmaceutical Sciences, USA
3Department of Clinical Sciences and Community Health, Italy
*Corresponding author: Cristina Maggioni, Humanitas, S. Pio X Obstetrics and Gynecology clinics Via Nava 31 Milano, Italy
Submission: April 01, 2020;Published: April 14, 2020

ISSN 2639-0531Volume2 Issue4
Introduction
Male factor infertility is the most common cause of involuntary childlessness [1,2]. It has been estimated that approximately 15% of reproductive-age couples suffer from infertility, which has become a global concern [3]. Nevertheless, in around 30%-40% of male infertility, no known cause is identified. Oxidative stress is an important factor for development of male infertility. During normal metabolism reactive oxygen species (ROS) produced within the respiratory chain of the mitochondria have the capacity to oxidize and damage cellular constituents such as lipids, proteins and DNA. Spermatozoa are more susceptible cells to oxidative stress than other cells since they have limited concentration of ROS-suppressing antioxidants due to the limited amount of cytoplasm in a mature sperm as well as high levels of unsaturated fatty acids in the sperm structure in the structure to protect plasma membrane surrounding acrosome and tail [4]. High rates of cellular proliferation increase mitochondrial oxygen consumption in testicular tissue. Additionally, exposure to X-rays, toxins and chemicals found in the environment, such as, cadmium [5], led [6,7], cigarettes [8] as well as specific physical conditions such as varicocele [9] can exacerbate oxidative stress and induce apoptosis of germ cells, affecting spermatogenesis. Increased production of ROS induces lipid peroxidation in spermatozoa, which has two important effects: Reducing sperm combination with oocyte and Increasing spermatozoa ability to bind to the transparent area (zona pellucida) [10].
As well, lipid peroxidation cause abnormality in the middle section of sperm and loss of acrosome capacity of fertilization. Oxidative stress causes increase in DNA breakdown and fragmentation of DNA [11] is commonly seen in infertile people’s spermatozoa, is due to the ROS high concentration in the sperm [12]. Pollen has traditionally been used for virility enhancement among males in various civilizations (e.g. Ancient Egypt and 200 BC China). Pollen and honey have been suggested to improve male infertility [13,14] and have been reported to have antioxidant, antimutagenic, anti-inflammatory and immunoregulatory properties. Pollen may interfere with multiple cell signaling pathway targets including those regulating: caspase induction in apoptosis; stimulation of TNF-α, IL-1β, IFN-γ, IFNGR1, p53, and immune cells; inhibition of lipoprotein oxidation, IL-1, IL-10, COX-2, LOXs, and PGE2; and modulation of other diverse targets. Pollen is rich in amino acids, vitamins, iron, calcium, minerals and nitric oxide. Specific components of pollen include superoxide dismutase (SOD) the main radical inhibiting free enzyme and tryptophan, a precursor to serotonin and fatty acids among other components. Previous data indicate that date palm pollen seems to cure male infertility by improving the quality of sperm parameters [13] but the results are difficult to interpretate because the chemical composition of pollen depends strongly on the plant source and geographic origin, together with other factors such as climate, soil type, and pollinator species and activities. Problematically, pollen can be of unspecific or unknown origin and may be mixed with other bee products making it difficult to standardize [15]. In addition, when the grain is intact, its components can be partially assimilated because the pollen shell cannot be damaged by stomach acid. The shell proteins are meant to be highly allergenic [16,17]. We use a unique, proprietary, technology for the manufacturing of a Purified and Specific Cytoplasmic Pollen Extract called FertiCyTonin® that involves destruction of the allergenic components of pollen which are broken down to peptides and amino acids. The process makes it possible to exclude any allergens, making purified and specific cytoplasmic pollen extract safe to use. In addition, the exclusion of the shell makes the active compounds highly bioavailable. In the final formulation CREALYS®®, contain purified and specific cytoplasmic extracts of pollen FertiCyTonin® (320mg). We add Zinc (10mg) and vitamin E (10mg), to avoid deficiency state of these elements that could interfere with FertiCyTonin® effect. SOD activity needs Zinc for his function. Moreover, Zinc plays a role in testicular development and sperm maturation and is correlated with hypogonadism and incomplete development of sexual characteristics in humans. Low levels of Zinc in semen have been associated with a reduction in fertilization capacity. Vitamin E (α-tocopherol) is a potent, lipophilic antioxidant, which is needed to protect and maintain mammalian sperm. Additionally, this element contributes greatly to the activity of Sertoli cell lines and spermatocytes.
We test the efficacy of CREALYS®®, by in vitro test and in vivo studies.
- Antioxidant activity in the DPPH2 (2-diphenyl-1-picrylhydrazyl) assay
- In vitro studies on NQO1,(NAD(P)H dehydrogenase quinone) gene induction is an enzyme that in humans is encoded by the NQO1 gene this gene is a member of the NAD(P)H dehydrogenase (quinone) family NQO1, stabilizes p53, protecting it from degradation, prevents reduction of quinones that results in the production of radical species and increase detoxifying agent
- Clinical studies on male infertile subject’s semen quality was be evaluated according to WHO criteria before and after the treatment followed for three months. Tolerance –Satisfaction-Compliance was also assessed.
Methods
In vitro studies
DPPH assay (2,2-diphenyl-1-picrylhydrazyl): 1mg/mL FertiCyTonin® in ethanol was prepared, vortexed vigorously for 10min, then sonicated via water bath for 2.5hrs. Solution was vortexed another 10min before serial dilution in ethanol to final concentrations. 0.1M DPPH in ethanol was prepared and stored in the dark at 4 °C until use. DPPH was warmed to RT before vortexing and plating. Wells were prepared in the dark as follows: blanks- 143uL sample + 57uL ethanol; negative control- 57uL DPPH + 143uL ethanol; positive control- 57uL ethanol + 143 ascorbic acid; pollen sample- 57uL DPPH + 143uL diluted sample. Wells were mixed by pipette then plate was placed in the dark for 1hr. Absorbance at 518nm was read on a Biotek SynergyMx. Antioxidant Activity (%) was calculated according to Mensor et al. [18].
Cell lines
SHSY5Y cells (ATCC, Manassas, Virginia, USA) were maintained in a mixture 1:1 of Ham's F12 and Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50U/mL penicillin and 50µg/mL streptomycin (0.5% P/S) in a humidified atmosphere of 5% CO2 in air at 37 °C. The cell medium was replaced every 3days. Cells were transferred to 6-well plates (seeded at 1e5 cells in 2mL media per well) for RNAseq experiments. At ~50% confluence, the medium was changed to DMEM: F12 supplemented with 1% FBS, 0.5% P/S, and 10 µM all-trans-retinoic acid (ATRA) for 3 days to initiate differentiation. Cells were then treated for 24 hours with 400 µg/mL FertiCyTonin® in maintenance medium.
RNA isolation
RNA from the cells treated as described above (groups in quadruplicate: control and 400µg/mL) was isolated using the Macherey-Nagel Nucleo Spin RNA kit as per the manufacturer’s instructions and resuspended in RNase-free water.
mRNA-seq profiling
The purity and concentration of the RNA extracts were measured on an Agilent Bioanalyzer (Agilent Technologies, Palo Alto, California, USA). A total of 200-500ng of total RNA was used to prepare Illumina HiSeq libraries according to the manufacturer’s instructions for the TruSeq Stranded RNA kit (Illumina Inc, San Diego, California, USA). The mRNA template libraries were then sequenced as single pass 2´150-bp reads on the Illumina HiSeq4000 platform at the University of Colorado’s Genomics and Sequencing Core Facility. A custom pipeline was used for analysis of the sequence data, as follows: Fast QC (v0.11.3) → Trimmomatic (v0.38) → Fast QC → HiSat2 (v2.1.0) → Sam Tools (v1.8) → Stringtie (v1.3.4d) → DESeq2 (v1.20.0) → BiomaRt (v2.36.1). Reads were mapped to the H. sapiens GRCh38 genometran index provided by the developers of HiSat2. Fold-change and false-discovery rate were calculated using DESeq2, and gene names were annotated using BiomaRt.
Clinical study
Clinical healthy men aged 20-50 years suffering for infertility since at least 2 years volunteer to enter the study. Exclusion criteria were azoospermia, excretory infertility, diabetes, oral treatment for hypertension, gastric ulcer, depression or anti-testosterone therapies. Men affected by actual prostatitis or infections were also excluded. All subjects signed the consent form. 20 consecutives subjects who met the inclusion criteria were treated with a daily dose of CREALYS® orally for a period of at least three months. Semen quality was evaluated according to WHO criteria [19] before and after the treatment followed Tolerance –Satisfaction-Compliance was also assessed.
Results
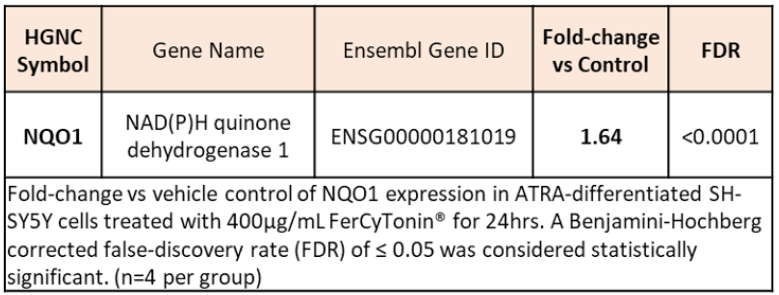
FertiCyTonin® pollen extract in CREALYS® exhibit antioxidant activity. DPPH is a free radical, stable at room temperature, is reduced in the presence of an antioxidant molecule. The use of DPPH provides an easy and rapid way to evaluate antioxidants. Based on results obtained from data in Figure 1 we can say that FertiCyTonin pollen extracts showed antioxidant activity. FertiCyTonin® pollen extracts in CREALYS® induce antioxidants genes. To further investigate and better understand which pathways were modulated by purified and specific cytoplasmic pollen extract and its correlation with the beneficial effect on sperm quality, a gene expression study was done in neurons. Human SH-SY5Y cells differentiated by retinoic acid (RA) has long been used to study neuronal signaling pathways modulated by chemical entities. RA treatment induces differentiation of these proliferating neuroblastoma cells to cells with a cholinergic neuron phenotype exhibiting neuron-like properties including neurites and cholinergic neuronal markers. In this study, mRNA extracted from cell treated with purified and specific pollen extract was analyzed by mRNA-seq profiling. Preliminary results show several modulated genes that may be correlated with the beneficial effects induced by purified and specific cytoplasm pollen extract in menopausal women. Among them, expression of NQO1, a member of the NAD(P)H dehydrogenase (quinone) family, was significantly induced. NQO1 stabilizes p53, protecting it from degradation, prevents reduction of quinones that results in the production of radical species and increases detoxifying agents (Figure 2).
Figure 1: FertiCyTonin exerts antioxidant activity.
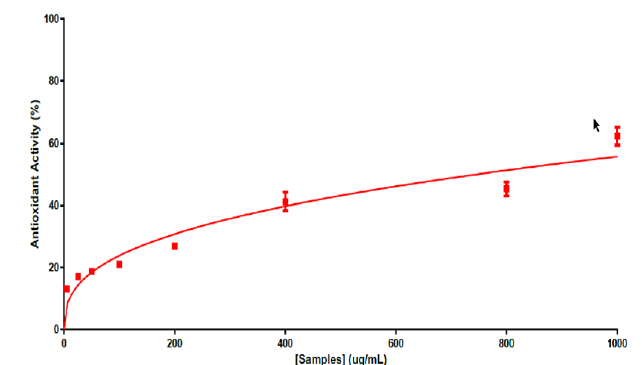
Figure 2: NQO1 mRNA induction by pollen extracts FertiCyTonin.
Clinical study
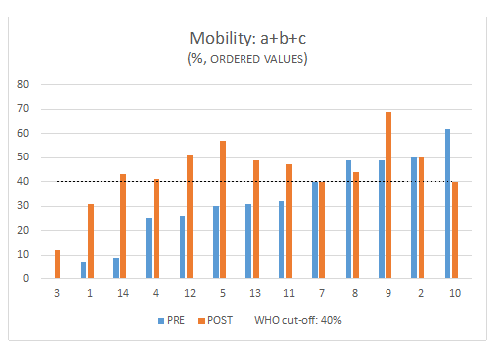
The treatment significantly increases sperm count, motility, and forward progressive motility. Mobility a and b: increase in 67% Mobility: a+b+c increase in 70% Sperm concentration increase in 77% Morphology did not show differences, (increase in 40%), globally all subject had an improvement in at least one parameter (Figure 3 & 4). Total motile sperm count (TMSC=Sperm concentration x Volume x Mobility a+b/100) was increased in 70% of subjects (Figure 5 & 6). We obtained 3 pregnancies. No side effects have been reported. 1 subject was excluded because older age, 1 drop out and the 4 are ongoing. In the sample that completed the study, 3 case had a decrease in total sperm count (but an increase in motility and total motility even higher that 100% , one of the case conceived after 5 years of infertility ) (Figure 7) and in 3 cases a decrease in motility but an increase in count.
Figure 3: Change after the treatment after 3 months of treatment with CREALYS.
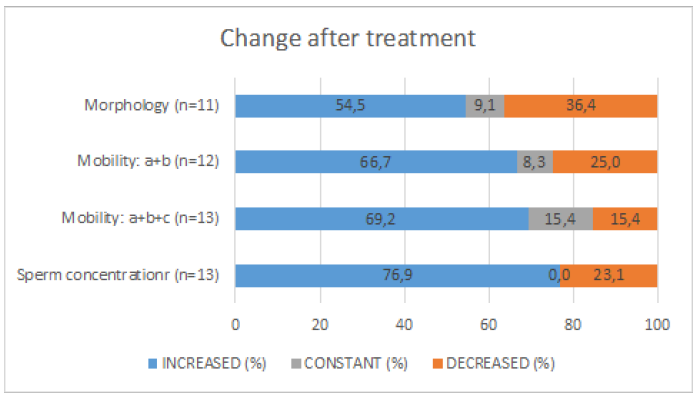
Figure 4: Median differences between pre-post treatment and 95% confidence intervals in Sperm concentration, Mobility: a+b+c, Mobility: a+b and Morphology.

Figure 5: Total motile sperm count (TMSC=Sperm concentration x Volume x Mobility a+b/100).
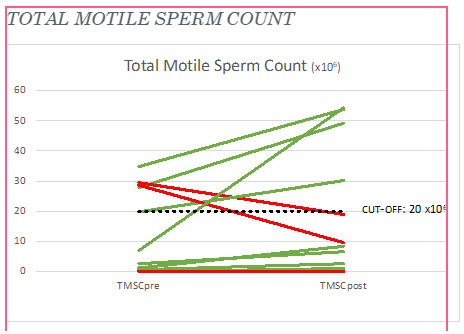
Figure 6: Percentage increase from pre to post treatment. Patient are ordered from the smallest to the highest value pre-treatment.
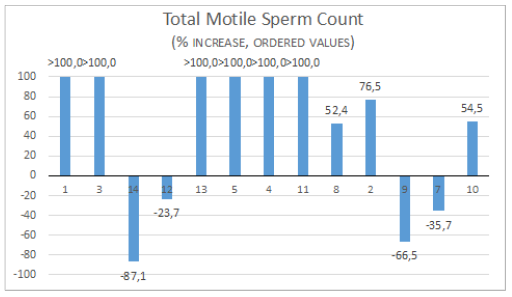
Figure 7: Mobility a+b+c (%) pre and post treatment and WHO cut-off. Patient are ordered from the smallest to the highest value pre-treatment.
Discussion
The possibility that an abnormal semen at first analysis eventually show a normal sperm count when more samples are examined is considered about 10% [20]. CREALYS® treatment induced a positive change in at least one sperm parameter in all subjects. Total motile sperm count (TMSC) index [21] is considered to have a better predictive value for spontaneous ongoing pregnancy than sperm parameters alone show an increase after CREALYS® treatment in 70% of cases. TMSC increment was higher than 100% the basal level in 46% case. This extraordinary improvement in sperm motility after 3 months treatment could be explained by the fact we did not set a cutoff for enter the study and even very compromised cases were included, the results show that the best results were obtained in very poor cases. A possible mechanism by which CREALYS® improve quality of sperm we suggest is the capacity to increase the antioxidant sperm capacity.
We know that the health and fertility of sperm are greatly dependent on the availability of the antioxidants, [22] which mostly is related to the antioxidant systems in the seminal plasma [23,24]. The body’s antioxidant defense system, called preventive antioxidant system, is related to antioxidant enzymes such as Superoxide Dismutase (SOD), catalase, and Glutathione Peroxidase (GPX). The importance of SOD is demonstrated in all aging processes and promote cellular senescence [24]. In a study on pine pollen it was reported on effect on SOD [25,26]. Honey are reported to have strong antioxidant activity [27,28]. It’s possible that other mechanisms of action, such as antimicrobial and antiviral activity, could account for these results [29-31]. Complementary and alternative medicine teachings believe that honey improves sperm quality in men [32]. To verify the antioxidant activity of the FertiCytonin, an in vitro DPPH antioxidant assay was used. DPPH assay as well described by Worachartcheewan et al. [33] and many others show the capacity of scavenging free radicals for a natural product. The NQO1 gene contains antioxidant response elements (ARE) in its promoter region and is regulated by the nuclear factor (erythroid- derived)-like 2 (Nrf2) [33]. The NQO1 gene has been shown to be activated together with other Nrf2-induced detoxifying enzyme genes, such as GST (glutathione S-transferase) and HO-1 (heme oxygenase), in response to antioxidants, ionizing radiation, xenobiotics, heat shock, electrophiles, hypoxia, and heavy metals [34]. The Nrf2/ARE signaling pathway is a crucial antioxidant pathway that is widely distributed in testicular cells and throughout the body. Cadmium treatment is known to decrease Nrf2 and its regulated mRNA and protein expressions of NQO1, HO1, γGCS, and GPx and increases Keap1 mRNA- and protein-expression levels in testis. Furthermore, exogenous Cd can suppress the intrinsic antioxidant capacity of testicular cells by inhibiting expression levels of Nrf2 and its regulated genes and thereby induce oxidant stress [35,36].
CREALYS® improve the quality of sperm by the antioxidant capacity and by the induction of antioxidant genes as NOQ1 gene. A possible mechanism of CREALYS® may be by activation of the anti-aging gene Sirtuin 1 (Sirt 1) since NQO1 binds and supports SIRT1 function [37]. The interaction of NQO1 with SIRT1 is markedly increased under mitochondrial inhibition. SIRT1 and NQO1 form a regulatory loop where SIRT1 regulates NQO1 expression and NQO1 binds and mediates the protective role of SIRT1. The balance between an NADH oxidoreductase enzyme and an NAD+ dependent deacetylase may depend on mitochondrial stress [37,38]. In our study, we did not observe an increase in SIRT1 gene expression, but CREALYS could activate SIRT1 protein activity without changing its basal mRNA expression. Investigation using a SIRT1 deacetylase assay [39,40] could be used to determine whether CREALYS displays any SIRT1 activity. Moreover, Sirt 1 deacetylates p53 [40] which is important for sperm concentration [41].
The results of the present study showed that Pollen extracts in CREALYS® induce significant expression of NQO1 gene which can contribute to the antioxidant pathway and consequently improve spermatogenesis. Further studies should be done to evaluate the impact of CREALYS® in cadmium toxicity or other situations as xenobiotics, heat shock, electrophiles, hypoxia, and heavy metals that leads to fertility. In the developing world CREALYS® would be appropriate with relevance to Sirt 1 repression and the high xenobiotic and xenometal levels.
Conclusion
CREALYS® product improves male infertility, by increasing sperm motility and number, acting on antioxidant and detoxifying semen capacity. A possible mechanism by which CREALYS® improve quality of sperm is the antioxidant capacity as shown in the DPPH assay and confirmed by the induction of antioxidant genes as NOQ1 gene. However further studies should be done in order to elucidate the exact mechanism of action. The extraordinary improvement in sperm motility after 3 months treatment indicates that CREALYS® may be a valuable tool to treat male infertility. More studies on a larger population are needed to confirm these clinical results.
References
- Ayala C, Steinberger E, Smith DP (1996) The influence of semen analysis parameters on the fertility potential of infertile couples. J Androl 17: 718-725.
- Boivin J, Bunting L, Collins JA, Nygren KG (2007) International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22(6): 1506-1512.
- Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, et al. (2002) Best practice policies for male infertility. Fertil Steril 77(5): 873-882.
- Aitken RJ, Clarkson JS (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fert 81(2): 459-469.
- Koizumi T, Li ZG (1992) Role of oxidative stress in single-dose, cadmium-induced testicular cancer. J Toxicol Environ Health 37(1): 25-36.
- Hsu PC, Liu MY, Hsu CC, Chen LY, Leon-Guo Y (1997) Lead exposure causes generation of reactive oxygen species and functional impairment in rat sperm. Toxicology 122 (1-2): 133-143.
- Marchlewicz M, Wiszniewska B, Gonet B, Baranowska-Bosiacka I, Safranow K, et al. (2007) Increased lipid peroixdation and ascorbic acid utilization in testis and epididymis of rats chronically exposed to lead. Biometals 20(1): 13-19.
- Mattison DR (1982) The effects of smoking on fertility from gametogenesis to implantation. Environ Res 28(2): 410-433.
- Smith R, Kaune H, Parodi D, Madariaga M, Rios R, et al. (2006) Increased sperm DNA damage in patients with varicocele: Relationship with seminal oxidative stress. Hum Reprod 21(4): 986-993.
- Said TM, Agarwal A, Sharma RK, Thomas AJ, Sikka SC (2005) Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta-nicotinamide adenine diy nucleotide phosphate. Fertil Steril 83(1): 95-103.
- Dorostghoal M, Kazeminejad SR, Shahbazian N, Pourmehdi M, Jabbari A (2017) Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia 49(10): e12762.
- Bennetts LE, Aitken RJ (2005) A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol Reprod Dev 71(1): 77-87.
- Rasekh A, Jashni HK, Rahmanian K, Jahromi AS (2015) Effect of Palm Pollen on Sperm Parameters of Infertile Man. Pak J Biol Sci 18(4):196-199.
- Banday MN, Lone FA, Rasool F, Rather HA, Rather MA (2017) Does natural honey act as an alternative to antibiotics in the semen extender for cryopreservation of crossbred ram semen? Iran J Vet Res 18(4): 258-263.
- Komosinska-Vassev K, Olczyk P, Kazmierczak J, Mencner L, Olczyk K (2015) Bee pollen: Chemical composition and therapeutic application. Evid Based Complement Alternat Med.
- Bogdanov S (2014) Pollen: production, nutrition and health: a review. Bee Product Science.
- Rimpler M (2003) Bleed pollen collected by bees: Properties and use of the medical journal Naturheilver-Fahren 3: 158-165.
- Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TCD, et al. (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15(2): 127-130.
- World Health Organization (2010) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. In: (5th edn), Cambridge: Cambridge University Press, USA.
- Vander Steeg JW, Steures P, Eijkemans MJC, Habbema JDF, Hompes et al. (2011) Role of semen analysis in sub-fertile couples. Fertil Steril 95(3): 1013-1019.
- Hamilton JA, Cissen M, Brandes M, Smeenk JM, De Bruin JP, et al. (2015) Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum Reprod 30(5): 1110-1121.
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ (2011) Antioxidants for male subfertility. Cochrane Database Syst Rev 19(1): CD007411.
- Velarde MC, Flynn JM, Day NU, Melov S, Campisi J (2012) Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging 4(1): 3-12.
- Mates JM, Sanchez-Jimenez F (1999) Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci 4: 339-345.
- Mao X, Zheng LD, Cao YB, Chen ZM, Lv YD, et al. (2012) Antiaging effect of pine pollen in human diploid fibroblasts and in a mouse model induced by D-galactose. Oxid Med Cell Longev.
- Lee KH, Kim AJ, Choi EM (2009) Antioxidant and antiinflammatory activity of pine pollen extract in vitro. Phytother Res 23(1): 41-48.
- Ahmed S, Sulaiman SA, Baig AA, Ibrahim M, Liaqat S, et al. (2018) Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid Med Cell Longev.
- Ahmed S, Othman NH (2013) Honey as a potential natural anticancer agent: a review of its mechanisms. Evid Based Complement Alternat Med.
- Bentrad N, Gaceb-Terrak R, Benmalek Y, Rahmania F (2017) Studies on chemical composition and antimicrobial activities of bioactive molecules from date palm (phoenixdactylifera L) pollens and seeds. Afr J Tradit Complement Altern Med 14(3): 242-256.
- Lee KH, Kim AJ, Choi EM (2009) Antioxidant and anti-inflammatory activity of pine pollen extract in vitro. Phytother Res 23(1): 41-48.
- Chen HJ, Wang ZP, Chen YR, Qin DS, Fu SJ, et al. (2002) Effects of pollen extract EA-10, P5 on chronic prostatitis or infertility with chronic prostatitis. Acta Pharmacol Sin 23(11): 1035-1039.
- Fakhrildin MB, Alsaadi RA (2014) Honey supplementation to semen-freezing medium improves human sperm parameters post-thawing. J Family Reprod Health 8(1): 27-31.
- Worachartcheewan A, Isarankura-Na-Ayudhya Ch, Prachayasittikul S, Prachayasittiku V (2011) Predicting the free radical scavenging activity of curcumin derivatives. Chemometrics and Intelligent Laboratory Systems 109( 2): 207-216.
- Kaspar JW, Jaiswal AK (2010) Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem 285(1): 153-162.
- Venugopal R, Jaiswal AK (1998) Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 17(24): 3145-3156.
- Xiaoxue Shi and Lijie Fu (2019) Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des Devel Ther 13: 2811-2824.
- Tsvetkov P, Adler J, Adamovich Y, Asher G, Reuven N, et al. (2017) NQO1 binds and supports SIRT1 function. Biorxiv.org.
- Kolthur-Seetharam U, Teerds K, Se Rooij DG, Wendling O, McBurney M, et al. (2009) The histone deacetylase SIRT1 controls male fertility in mice through regulation of hypothalamic-pituitary gonadotropin signaling. Biol Reprod 80(2):384-391.
- Borra M, Smith BC, Denu J (2005) Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280(17): 17187-17195.
- Dell’Omo G, Crescenti D, Vantaggiato C et al. (2019) Inhibition of SIRT1 deacetylase and p53 activation uncouples the anti-inflammatory and chemo-preventive actions of NSAIDs. Br J Cancer 120(5): 537-546.
- Mostafa T, Nabil N, Rashed L, Makeen K, El-Kasas MA, et al. (2018) Seminal SIRT1 expression in infertile oligoasthenoteratozoospermic men with varicocoele. Andrology 6(2): 301-305.
© 2020 Cristina Maggioni. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






