- Submissions

Full Text
Advancements in Case Studies
The Influence of Various Drags on Mortality of Mice and the Concentration of Proinflammatory Cytokines in Blood at Sepsis Caused by E. Coli
Zabrodskii PF*
Saratov Medical University “REAVIZ”, Saratov, Russian Federation
*Corresponding author: PF Zabrodskii, Saratov Medical University “REAVIZ”, Saratov, Russian Federation, Russia
Submission: March 22, 2018;Published: May 18, 2018

ISSN 2639-0531Volume1 Issue3
Abstract
Experiments on albino mice showed that administration m-cholinomimetic (aceclydine), n-cholinomimetic (nicotine), reversible inhibitor of acetylcholinesterase (neostigmine methyl sulfate), n-cholinomimetic α7nAChRs agonist (GTS-21), epinephrine hydrochloride, adenomimetic β2ARs agonist (hexaprenaline sulfate) causes decrease in mice mortality in sepsis caused by the administration (i.p.) of E. coli O157:H7 and the concentration of TNF-α, IL-1β and IL-6 in the blood in comparison with parameters at sepsis without use of drugs.
Keywords: Sepsis; Cytokines; E. coli; Cholinergic drugs; Adrenergic drugs
Introduction
From all the lethal outcomes associated with diseases and their complications, mortality from sepsis, including that caused by opportunistic Gram-negative microorganisms (E .coli, etc.) varies from 12 to 60% depending on various factors [1], and the frequency of lethality from it increases [2,3]. In 1987, a cholinergic anti-inflammatory mechanism was discovered [4], named in 2002 “cholinergic anti-inflammatory pathway” after the study of its realization at the organismal, cellular and subcellular levels [5,6,7]. It should be noted that in 1995 the possibility of using cholinomimetics for the immediate activation of antimicrobial resistance of the organism during sepsis was proved [5]. Further study of the cholinergic anti-inflammatory pathway caused by the action of acetylcholine on α7n-acetylcholinoreceptors (α7nAChRs) of cells of the monocyte-macrophage system, followed by inhibition of the production of these cells by pro-inflammatory cytokines TNF-α, IL-1β, IL-6, B1-HMGB1 protein, macrophage-inflammatory protein-2-MIP-2 and decrease in mortality from sepsis were devoted to hundreds of articles by different authors [8-11].
The cholinergic anti-inflammatory pathway is realized due to the activation of acetylcholine m-acetylcholine receptors type 1 (m1AChR) of the brain, modulating the immunoregulatory function of the vagus nerve; excitation of efferent fibers n. vagus; the action of acetylcholine on α7nAChRs cells of monocyte-macrophage system [3,6,7,12,13]. In cells of the monocyte-macrophage system, the anti-inflammatory effect is provided by the kinase JAK2, the transcription factor STAT3, the transcription factor NF-κB (nuclear factor kappa B, NF-kappa B) [3,6].
Along with the cholinergic anti-inflammatory pathway, there is an adrenergic anti-inflammatory mechanism [3], associated with sepsis, inflammatory bowel diseases and other infectious processes involving the activation of adrenal medulla and sympathetic ganglia n-cholinergic receptors, which leads to epinephrine and norepinephrine production, which by exciting adrenoreceptors of cells of monocyte-macrophage system [3,14], β2-adrenoreceptors (β2ARs) of spleen T-lymphocytes [3,9], cause the same effect as the activation of α7nAChRs, leading to reduction of synthesis of proinflammatory cytokines by cells of the monocyte-macrophage system [3,7,10].
The aim of the study was to study cholinergic and adrenergic drugs on mouse mortality in early phase of experimental sepsis caused by E. coli and the content of proinflammatory cytokines TNF-α, IL-1β, IL-6 in the blood.
Materials and Methods
The experiments were carried out on mongrel white mice of both sexes weighing 18-22g. The control group of mice (control group 1) received 0.5ml of isotonic sodium chloride solution (saline) 10-30 minutes later in 2.0ml of saline. The second group of mice (control group 2) was injected i.p., once with 0.5-1.0ml of saline. Fifteen to 60 minutes after the administration of saline, mice received 2.5×109 CFUs in 2.0ml of saline diurnal culture of E. coli O157:H7 (modeling of sepsis) [3,4,5,15]. All the drags (groups of mice 3-9) were administered (i.p., a single) in 0.5-1.0ml of saline.
M-cholinomimetic aceclydine (Vector HRC of Virology and Biotechnology, Russia) (3rd group of mice) penetrating the bloodbrain barrier, n-cholinomimetic nicotine (Sigma-Aldrich) (4th group), reversible acetylcholine esterase inhibitor-neostigmine methyl sulfate (Sigma-Aldrich) (5th group) was administered at dose of 0.3 LD50 (LD50 data drags were for mice, respectively, 3.8±0.2, 30.0±2.5, 0.45±0.10mg/kg). The sixth group of mice received the n-cholinomimetic α7nAChRs agonist GTS-21 [3- (2,4-dimethoxybenzylidene)-anabaseine dihydrochloride] (Sigma-Aldrich) at a single dose of 5mg/kg [16]. Epinephrine hydrochloride (Sigma-Aldrich) 0.5mg/kg (group 7) and selective β2ARs agonist (8th group) dexmedetomidine hydrochloride (Orion Pharma) were used as adrenergic drugs, which was administered at a single dose of 25 μg/kg [17]. In groups 3 and 8, 1.0-2.0 h after the administration of the drugs, sepsis was modeled. The registration of the lethality of mice (groups 2-8) was performed 6 and 24 hours after the modeling of sepsis.
The concentration of TNF-α, IL1β and IL-6 was studied in blood plasma of all groups of mice (groups 1-8) 6 and 24 hours after the administration of E. coli (sepsis modeling) by enzyme immunosorbent assay (ELISA) using kits (ELISA Kits MyBioSoure) in accordance with the manufacturer’s instructions. Monoclonal antibodies MyBioSoure (TNF-α, IL1β, IL-6 - #MBS494184, #MBS494492, #MBS335516) were used to determine the concentration of pro-inflammatory cytokines. Blood for research was taken from the retroorbital venous sinus. The data obtained were processed statistically using the Student’s t-test. Differences between the parameters were considered reliable at p<0.05.
Results
Table 1: Effect of cholinergic and adrenergic drugs on mortality of mice with E. coli-induced sepsis (M±m).
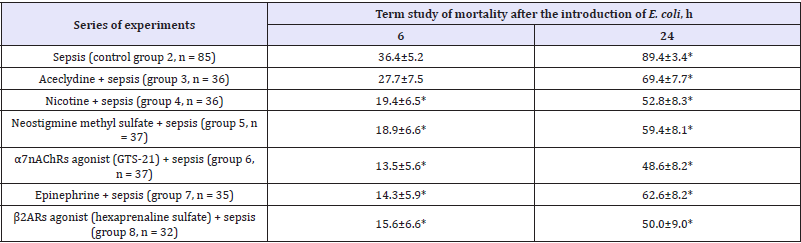
Table 2: Effect of cholinergic and adrenergic drugs on the concentration of pro inflammatory cytokines in blood of mice with E. coli sepsis, pg / ml (M ± m; n = 7-9).

The use of m-cholinomimetic aceclydine, n-cholinomimetic nicotine, reversible inhibitor of acetylcholine esterase (neostigmine methyl sulfate), n-cholinomimetic agonist α7nAChRs (GTS-21), epinephrine, adrenergic agonist β2ARs (hexaprenaline sulfate) caused a decrease in mortality 6 hours after i.p. administration of E. coli compared to with the control group 2 (sepsis without the use of drugs), respectively, by 8.7 (p> 0.05); 17.0, 17.5, 22.9, 22.1 and 20.8 (p<0.05), and after 24hours - by 20.0, 36.6, 30.0, 40.8, 26.8 and 39.4 (p<0.05) (Table 1,2). The effect of aceclydine is less pronounced than that of other drugs (p> 0.05)
The results indicate that the applied drags, both cholinomimetics, and agonists reduced the mortality of mice from sepsis (administration of E. coli) approximately equally, the maximum effect was observed when applying α7nAChRs agonist (GTS-21), minimal effect - when using m-cholinomimetic aceclydine. The results obtained suggest that the decrease the mortality of mice in the modeling of sepsis with administration of E. coli (i.p.) after the use of a reversible inhibitor of acetylcholine esterase (neostigmine methyl sulfate) and n-cholinomimetic (nicotine) is due to activation of acetylcholine (action of neostigmine methyl sulfate), central m-cholinergic receptors by aceclylidine, and activation of α7nAChRs and β2ARs cells monocyte-macrophage system [3-5,18-20]. It should be noted that in large doses aceclydine activates probably not only the central m1AChRs [6,14], but and the α7nAChRs.
The concentration in the blood of TNFα, IL1β and IL-6 mice after the modeling of sepsis (administration of E. coli i.p.) after 6 hours compared with the control (group 1) increased by 12.1, 20.3, 27.1 times (p<0.05). For 24 hours, the blood TNFα content was significantly reduced (p<0.05), reaching almost the control level, and the concentration of IL1β and IL-6 exceeded the control parameters by 4.8 and 8.5 times, respectively (p<0.05).
After the use of aceclydine, nicotine, neostigmine methyl sulfate, n-cholinomimetic agonist α7nAChRs (GTS-21), epinephrine, adrenomimetic β2ARs agonist (group 2-8), followed by modeling of sepsis (administration of E. coli) concentration of TNF-α in the blood of mice decreased in 6 hours compared to parameters for sepsis without the use of drags (control group 2), respectively, at 59.1, 70.8, 74.4, 77.9, 71.9 and 75.1% (p<0.05); IL1β concentration decreased by 42.6, 61.2 , 63.3, 67.0, 60.0 and 63.1% (p<0.05), and the concentration of IL- 6 - by 78.0, 85.2, 86.4, 89.9, 88.3 and 88.9% (p<0.05), respectively. 24 hours after the administration of E. coli, the content of TNFα in the blood after the application of cholinergic and adrenergic drags in sepsis did not practically differ from the parameters of control group 1 (intact mice) and group 2 (sepsis without drags), IL1β concentration with m-cholinomimetic aceclydine decreased by 31.9% (p<0.05) compared with the parameter in group 2 (sepsis), and in the case of other drugs - an average of 60.0% (p<0.05). The concentration of IL-6 in 24 after the administration of E. coli decreased by 46.0% (p<0.05) compared with the parameter in group 2 (sepsis) with the use of aceclydine, and by 67.2% on other drugs (p< 0.05).
It should be noted that, on average, the reduction of the concentrations of TNFα, IL1β and IL-6 6 hours after the administration of E. coli with the use of aceclydine (59.7%) compared to other drags (decrease of 74.8% on average) was 15.1% less is expressed (p<0.05).
The literature data suggest that the reduction of synthesis of proinflammatory cytokines TNFα, IL1β and IL-6 after administration of aceclydine in sepsis is due to its effect on m1AChR of the brain [6,9,21] and subsequent activation of the cholinergic antiinflammatory pathway. Effects of nicotine, acetylcholine esterase inhibitor neostigmine methyl sulfate, n-cholinomimetic α7nAChRs agonist (GTS-21) are associated with the activation of α7nAChRs cells of the monocyte-macrophage system of liver, gastrointestinal, and spleen nAChRs [20,21]. Reduction of production of TNFα, IL1β and IL-6 under the influence of epinephrine, adrenomimetic agonist β2ARs (hexaprenaline sulfate) occurs as result of direct and indirect (through β2ARs T spleen T cells) activation of monocyte-macrophage system cells [3,9]. It is known that monocytes and macrophages have βARs, and their activation leads to an anti-inflammatory effect [14] due to inhibition of the nuclear transcription factor NF-κB [22].
Thus, n-cholinomimetics, reversible inhibitors of acetylcholinesterase, adrenomimetics and m-holinomimetics can be considered as promising drugs, along with other drugs, for the treatment of septic conditions, inflammatory bowel diseases and other infectious diseases caused by opportunistic Gram-negative microorganisms.
Conclusion
1. The use of m-holinomimetic (aceclydine), n-cholinomimetic (nicotine), reversible inhibitor of acetylcholinesterase (neostigmine methyl sulfate), n-cholinomimetic α7nAChRs agonist (GTS-21), epinephrine hydrochloride, adenomimetic β2ARs agonist (hexaprenaline sulfate) causes a decrease in mice mortality in sepsis caused by the administration i.p. of E. coli O157:H7.
2. Administration to mice of m-cholinomimetic aceclydine, nicotine, neostigmine methyl sulfate, α7nAChR agonist (GTS- 21), epinephrine hydrochloride, adrenomimetic β2ARs agonist (hexaprenaline sulfate) practically simultaneously with modeling of sepsis, decreased blood concentrations of TNF-α, IL-1β and IL-6 compared with parameters at sepsis without the use of drugs.
3. M-cholinomimetic acetylidine compared with the effects of nicotine, the reversible cholinesterase inhibitor neostigmine methyl sulfate, the α7nAChRs agonist (GTS-21), epinephrine hydrochloride, adrenomimetic β2ARs agonist (hexaprenaline sulfate) are more pronounced.
References
- Internet Pharmacy warning letters, U.S. Food and Drug Administration, Updated on February 2, 2017. https://www.fda.gov/Drugs/DrugSafety/ DrugIntegrityandSupplyChainSecurity/ucm348680.htm Accessed on May 5, 2017.
- Orizio G, Merla A, Schulz PJ, Gelatti U (2011) Quality of Online Pharmacies and Websites Selling Prescription Drugs: A Systematic Review. J Med Internet Res 13(3): e74.
- Brushwood DB (2001) Responsive regulation of Internet pharmacy practice. Annals of Health Law 10: 75-103.
- Aggarwal KB (2016) Need to amend Drugs and Cosmetics Act 1940 to promote e-pharmacy in India, FICCI Media Division.
- Will online pharmacies work in India, and are they even legal? www. livemint.com, Modified on January 14, 2016. https://www.livemint. com/Companies/Will-online-pharmacies-work-in-India-and-are-theyeven- legal.html Accessed on February 10, 2016.
- The possible dangers of buying medicines over the internet, U.S. Food and Drug.
© 2018 Zabrodskii PF. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






