- Submissions

Full Text
Annals of Chemical Science Research
Determination Of Optimal Conditions for Obtaining Shot from Houshoil Metal Waste
Ibrahima Sory Sow1*, Dong Yang2 and Amadou Youssouf Bah1
1Physical and inorganic chemistry laboratory, Faculty of Science, Université Gamal Abdel Nasser de Conakry (UGANC), Guinea
2Clinical laboratory, Shanxi Provincial People’s Hospital, Affiliated of Shanxi Medical University, China
*Corresponding author:Ibrahima Sory Sow, Physical and inorganic chemistry laboratory, faculty of science, Université Gamal Abdel Nasser de Conakry (UGANC), BP 1147 Conakry, Guinea
Submission: February 07, 2024;Published: April 03, 2024

Volume4 Issue4April , 2024
Abstract
Six types of cans were used to obtain the shot (sardine, tomato, milk, varnish, paint and juice). Parameters of time, temperature and reaction media (HCl, NaOH, seawater and tap water) were screened as the optimal physical parameters in the experiments. Heated to 500 °C and introduced into the NaOH solution (0.2mol/L), shot was obtained after a six-month waiting period from juice cans. Increasing the temperature at 1080 °C, the shot was extracted with the five other types of metal cans used. This research showed that the temperature and basic medium were the optimum conditions for obtaining shot. It is deserved t to study the chemical composition of the metal cans in greater depth, with a view to use in the agriculture or craft industry in the future.
Keywords:Shot; Optimal conditions; Metallic rejets
Introduction
The metal cans made of non-toxic metals (iron, tin, aluminium) are used to preserve food products and the cans made of toxic metals (lead cadmium) or zinc are sorted non-food [1]. In Conakry (Guinea), residents usually burn various cans to get rid of the houshoil waste. However, these behavious of burnt rubbish to produce the toxic products which are not only harmful to human but also contribute to environmental pollution.
The recycling system could recycle some useful productors from solid waste used in crafts, agriculture and industry. For example, recycling 1kg of aluminium can save 8kg bauxite, 4kg chemicals (carbon, aluminium fluoride and cryolite) and 13-15kWh electricity [2,3]. The aluminium recycling also saves up to 95% energy to produce the primary metal [3]. Indeed, the recycling system saves energy, reduces waste and provides additional income for recyclers. In addition, it is well known that metals are present in soils with various chemical forms, which influence the reactivity and hence the mobility and bioavailability [4].
In Guinea, recycle system was not well developed and the priority of houshoil waste were collected and transferred to inappropriate landfill sites. In the present study, we investigated the optimal conditions to recycle the cans from houshoil wastes. One hand, it could be a drive to recycle metal cans. The other hand, using a simple technique could be used to enrich soils that are poor in chemical elements. It’s well know that the plants take up iron from the soil. Iron is an essential element for plants as it is involved in chlorophyll biosynthesis and photosynthesis [5].
Experimental Section
Materials
Metal cans (tomato, sardine, milk, paint, varnish and juice). Nabertherm oven (Schalt model), dessicator (Glaswerk Wertheim GL2), analytical balance (AC 120S, Sartorins), jars, pH meter, stopwatch, cutting pliers. NaOH (0.2mol/L) and HCl solutions (0.2mol/L), seawater, mains water, distilled water.
Methods
The metal cans were collected by the selective sorting system from the houshoil waste in the commune of Dixinn, Conakry. After cleaning, the cans were cutted into strips. A 5g slice can was heated to a temperature of 500 °C for 30min or 1080 °C for 60min. After removing from the oven, the slice cans were placed in a desiccator for 3 hours to cool. They were reweighed before being placed in the jars containing the reaction media. An unheated “control” slide weighing around 5g was inserted into each jar containing a heated slice of the same type. Previously heated slices were also exposed to open air for three months under laboratory bench conditions. The time elapsed for observation was 3 to 6 months.
Results and Discussion
Initially, the metal can strips were heated on a temperature below the melting point of the desired metals to elucidated that a temperature is less than 500 °C (Table 1). The melting points of iron, aluminium, tin, lead, zinc and cadmium are 1535 °C, 660 °C, 232 °C, 327 °C, 419 °C and 321 °C, respectively [6].
Table 1:The main information to obtain the shot from various cans.
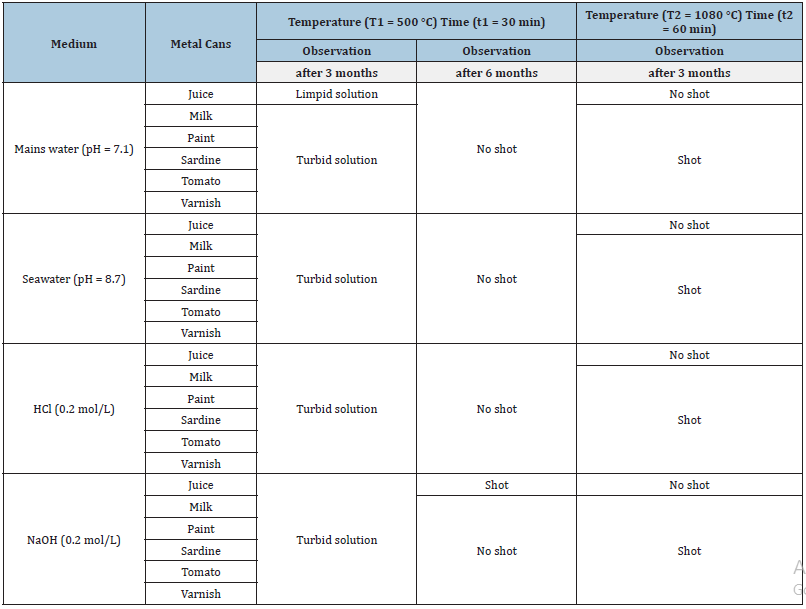
Secondly, in the 0.2mol/L NaOH solution, the shot obtained from juice cans heated to 500 °C (Table 1), confirming that it was made of aluminium alloys (e.g.: Al (99.7%), Fe and Si (0.3%)) [7]. It has been reported that remelting aluminium scrap only consumes 2.8kWh/kg due to its low melting point (660 °C) [8]. The shot can be obtained easily from juice cans in an alkaline solution.
Finally, if the temperature is higher than the melting point of the desired metals, it could be the optimal temperature obtaining the shot.
Conclusion
In this study, we were able to obtain shot from juice cans by heated to 500 °C and dissolved in a 0.2mol/L NaOH solution after 6 months. Without a reaction medium, it was possible to obtain shot from metal cans. e.g. sardines, tomatoes and varnish can, with a higher temperature of 1080 °C. Temperature is one the most important physical parameters for obtaining optimal shot from metal rejects. Obtaining shot from metal waste could help the authorities in Conakry to develop a simple and rapid system to recover houshoil waste.
Acknowledgements
Dr Abdoulaye Keïta and Hector Mahomy for their help in achieving experimental procedures and for providing some reagents.
Funding
This work was not funded.
Conflicts of interest
The authors declare no conflicts interest.
Author contributions
Conceptualization, Methodology, Analysis and Writing: I.S.S; Review and Editing: D.Y. and I.S.S.; Supervision and Validation: A.Y.B. Authors have read and agreed to the published version of the manuscript.
References
- Nasa P (2014) A review on pharmaceutical packaging material. World J Pharm Res 3(5): 344-368.
- Kvande H, Drabløs PA (2014) The aluminum smelting process and innovative alternative technologies. J Occup Environ Med 56(5 Suppl): 23-32.
- AlSaffar KA, Bdeir LMH (2008) Recycling of aluminum beverage cans. J Eng Technol 12: 157-163.
- Abollino O, Aceto M, Malandrino M, Mentasti E, Sarzanini C, et al. (2002) Heavy metals in agricultural soils from Piedmont, Italy. Distribution, speciation and chemometric data treatment. Chemosphere 49(6): 545-557.
- Kobayashi T, Nozoye T, Nishizawa NK (2019) Iron transport and its regulation in plants. Free Radic Biol Med 133: 11-20.
- Noohi Z, Nosouhian S, Niroumand B, Timelli G (2022) Use of low melting point metals and alloys (Tm < 420 °C) as phase change materials: A Review. Metals (Basel) 12: 945-974.
- Rivera NMT, Torres JT, Valdés AF (2019) A-242 aluminium alloy foams manufacture from the recycling of beverage cans. Metals (Basel) 9(1): 92-106.
- Raabe D, Ponge D, Uggowitzer PJ, Roscher M, Paolantonio M, et al. (2022) Making sustainable aluminum by recycling scrap: The science of “dirty” alloys. Prog Mater Sci 128: 100947.
© 2024 Ibrahima Sory Sow. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






