- Submissions

Full Text
Annals of Chemical Science Research
Immobilized Polyolefin Catalysts: Organic Versus Inorganic Supports
Xiong Wang1*, Guangquan Li1, Pinglin He2, Dewen Chen3 and Wenqian Kang1
1Lanzhou Petrochemical Research Center, Petrochemical Research Institute, PetroChina, China
2Polyolefin Operation Department II, Lanzhou Petrochemical Company, PetroChina, China
3Chemical Operation Department I, Lanzhou Petrochemical Company, PetroChina, China
*Corresponding author:Xiong Wang, Lanzhou Petrochemical Research Center, Petrochemical Research Institute, PetroChina, Lanzhou 730060, China
Submission: March 21, 2023;Published: March 29, 2023

Volume3 Issue5March , 2023
Abstract
The polyolefins are the most widely used synthetic resin and greatly influence nearly every walks of life. About 70% polyolefin production is made from the immobilized polyolefin catalysts in gas-phase, slurry or bulk process equipment. Although the inorganic supports have been successfully industrialized, some inconvenient drawbacks calls for a new type of support to solve those problems. Porous organic polymers (POPs) have proved to be an excellent choice and have been considered as a versatile platform for the deployment of catalysts. The flexible design and synthesis approach provides POPs with high surface area and bulk density, excellent flowability, and various functionalities for desired catalysts performance. The POPs-based polyolefin catalysts obtained high polymerization activity and tailored molecule structure including molecular weight, molecular weight distribution, chemical composition distribution, stereoregularity, etc. due to the nature of the organic support.
Keywords:Porous organic polymers (POPs); Olefin polymerization; Metallocene catalyst; Ziegler-Natta catalyst
Introduction
Since the discovery of Ziegler-Natta and Phillips catalysts, polyolefins have witnessed continuous and tremendous growth of production and consumption combined with polymerization process innovation. By far, about 70% of total commercial polyolefins are based on the supported catalysts and inorganic supports have been widely used in the immobilized polyolefin catalysts. The supported high yield catalysts are compulsorily used in gas-phase, bulk or slurry phase industrial polyolefin equipment, to avoid reactor fouling by the particle morphology control of catalysts without further atactic removal and purification [1,2].
A great deal of researches focusing on inorganic supports such as silica gel, magnesium dichloride, molecular sieves, aluminium oxide, zeolite and clays have been reported [3-7] for olefin polymerization. The inorganic MgCl2 and SiO2 have been successfully applied in the conventional Ziegler-Natta or metallocene catalysts. The prepared polymers obtained excellent particle morphology and flowability by replication effect of the inorganic supports especially with sphere or granule shape, which is suitable for gas phase or slurry process. Furthermore, the inorganic supports typically tend to be highly porous with active sites dispersed more or less evenly throughout them, thus benefiting the diffusion of monomer(s) to the surface of the active sites around the supports and helping the immobilized Z-N catalysts or metallocene catalysts to achieve high polymerization activity.
Despite obvious physical advantage and the tremendous success, these inorganic supports based polyolefin catalysts undergo several deficiencies. The excess of surface acidic groups, such as the silanol or -SiOH on the silica surface, could cause catalyst deactivation and need tedious pre-treatment before immobilization of active metal on their surface [8,9]. Due to inorganic residuals within the prepared polymer matrix during the process of catalysts fragmentation, the properties of the produced polyolefins could be harmed. For example, the mLLDPE film produced from a silica supported metallocene catalyst, more often than not, suffer from observable fisheyes which mainly caused by the inorganic SiO2 impurities and the inhomogeneous insertion of comonomer (1-hexene) in both high molecular weight and low molecular weight PE with significantly different melting temperature. Although the supports are generally considered as an inert composition during the polymerization, the high ash content of inorganic support-based catalysts restrict their application in high-end and cleaner areas, such as electrical film and medical appliance.
The search for green and environmentally friendly alternative is a major motivation for scientists or researchers, especially in electrical and medical industries. As a replacement, porous organic polymers (POPs) provide a much similar polymerization environment with homogeneous polymerization and incorporate into the polymer no extra impurities which would dramatically affect the properties of the final product [9,10], except for the active components. It’s very facile and feasible to design and synthesize functionalized POPs with desired polymer frameworks and surface chemistry, by a selection of suitable solvent and monomer and/ or functional monomers especially by radical polymerization, and the post-modification approaches also provide versatile routes for various functionality of the porous materials [11]. A schematic illustration of how the supports can influence the polymer molecular structure is presented in Figure 1.
Figure 1:Schematic illustration of how the supports can influence the polymer molecular structure..
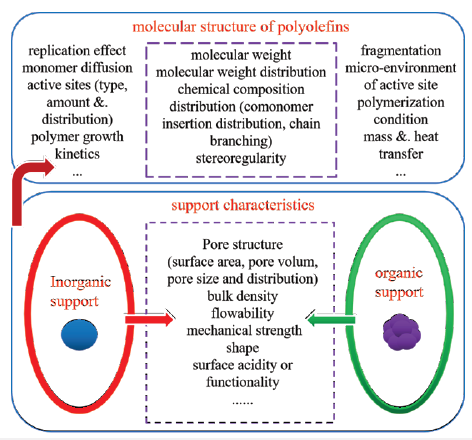
For those reasons, porous organic polymers have attracted lots of interests from academic and industrial researchers over the past several decades [12-18]. The high surface area combined with the well-defined pore structure and functionalities offer POPs as a versatile platform for the deployment of heterogeneous polyolefin catalysts. A series of studies on organic polymer-supported polyolefin catalysts were reported with functional group on the surface, and most of the synthesized porous organic polymer are based on styrene or divinylbenzene monomer. In order to anchor the active sites of Ziegler-Natta catalysts or metallocene catalysts, Functional comonomer(s) are involved in these polymers with acrylic acids, hydroxyl, amino, esters, chloromethyl, sulfonate groups, etc. For the POPs-based metallocene catalysts, they exhibits excellent ethylene homo-polymerization and ethylene/α- olefin copolymerization abilities and much higher polymerization activity than silica-based metallocene catalysts could be obtained. However, the polymer morphology and bulk density didn’t manifest to be as good as that of silica-based catalysts. Similar predicaments appeared in the POPs-based Ziegler-Natta catalysts, in addition, the polymerization activity was a little lower than the MgCl2-based counterparts, but acceptable for industrialization. It seemed that it is still a long time to go before successful commercialization for the organic polymer supported catalysts.
Given that the bad polymer morphology and low bulk density straits could be solved, the POPs-based polyolefin catalysts are promising. The feasibility of design and synthesis of POPs with high surface area, controllable pore structure, surface morphology and bulk density has been investigated by our group [19]. By selection of appropriate solvent(s) with matched thermodynamic solubility with used comonomer and the prepared polymer, template agent, functional monomer and cross ling agent content, the synthesis of eligible POPs which meet the requirement as the commercial polyolefin catalysts supports would be practicable, and POPs with high surface area, good surface morphology and high bulk density were prepared. In another attempt, highly flowable nano TiO2/ POPs hybrid supports with reasonable pore structure and surface morphology were synthesized and the prepared metallocene catalysts exhibited higher ethylene polymerization activity than inorganic silica supports with no obvious activity decay for 2 hours [20]. This inorganic-organic hybrid approach proved to be an efficient way to overcome the drawbacks, and will surely benefit to facilitate the industrialization of POPs in olefin catalysis.
Besides the importance of the support on activity, the POP support also can profoundly influence on the prepared catalysts and polymer properties. The organic support can remarkably change the surface hydrophilicity and modify the micro chemical environment of active sites on the catalyst, which would reflect in molecular weight, molecular weight distribution (MWD), chemical composition distribution (CCD) and stereoregularity (when propylene polymerization were conducted). As reported the sulfonated POPs-supported Ziegler-Natta catalysts for propylene polymerization by our group, the immobilized catalysts exhibited particular properties with broad molecular weight distribution and high stereoselectivity with common internal electron donor of diisobutyl phthalate (DIBP) adopted [21].
Klapper and co-workers [12] have revealed that the inorganic and organic supports based metallocene catalysts can impose substantial inconsistency in the incorporation of comonomer and short chain branching (SCB) [22]. The SCB distribution of the prepared PE copolymer from silica gel-supported metallocene is inhomogeneous, while by comparison, 1-hexene insertion is quite uniform in all the PE chains from the organic PS-based metallocene. Thus, homogeneous CCD of the copolymers are obtained and phase separation with different melting temperature due to branching distribution is limited, which is beneficial to the development of high-end mPE materials. The main explanation was proposed from a series of analysis that the monomer diffusion process plays a critical role in the incorporation of higher α-olefin. The rigid or flexible surface nature between the silica and organic supports cause different monomer diffusion behavior at the active sites on the surface and inside the silica pores. The formed crystalline polymer layer around the rigid surface of silica affects, in particular, the diffusion of 1-hexene with the bigger size, resulting in a broadening MWD and heterogeneous CCD. In contrast, the filtering effect of monomer diffusion is negligible due to the different fragmentation behavior for organic supports-based catalysts, and the ethylene/ comonomer ratio is similar at all active sites around the micrograin surface and the broadening phenomenon is suppressed [23].
Conclusion
In conclusion, Porous organic polymers (POPs) have showcased their enormous potential for industrial application as a new type of polyolefin catalyst supports. The high surface area, modifiable pore structure and functionalities, cleaner and greener chemical composition will endow the porous organic polymers with a versatile platform for various olefin polymerization catalysis. The change of support from inorganic to organic offers us a powerful and practical tool to tailoring the polymer properties along with varying catalyst types and polymerization process conditions. The unique aggregation and fragmentation modes of POPs occurring in the synthesis of the organic particles and polymerization process, respectively, facilitate homogeneous insertion of comonomers and uniform distribution of CCD and short chain branching, etc. Porous organic polymers present a new route to solve the emerging obstacles or drawbacks in the inorganic supports-based polyolefin catalysts.
Acknowledgment
The financial support of this work by PetroChina Company Limited is gratefully acknowledged.
References
- Galli P, Vecellio G (2004) Polyolefins: The most promising large-volume materials for the 21st century. J Polym Sci A Polym Chem 42(3): 396-415.
- Galli P, Vecellio G (2001) Technology: Driving force behind innovation and growth of polyolefins. Prog Polym Sci 26(8): 1287-1336.
- Aline C Dos Ouros, Michèle O De Souzab, Heloise O Pastore (2014) Metallocene supported on inorganic solid supports: An unfinished history. J Braz Chem Soc 25(12): 2164-2185.
- Trivedi PM, Gupta VK (2021) Progress in MgCl2 supported Ziegler-Natta catalyzed polyolefin products and applications. J Polym Res 28(45): 1-20.
- Tisse VF, Prades F, Briquel R, Boisson C, Mckenna TFL (2010) Role of silica properties in the polymerization of ethylene using supported metallocene catalysts. Macromol Chem Phys 211(1): 91-102.
- Kumkaew P, Wanke SE, Praserthdam P, Danumah C, Kaliaguine S (2003) Gas‐phase ethylene polymerization using zirconocene supported on mesoporous molecular sieves. J Appl Polym Sci 87(7): 1161-1177.
- Meshkova IN, Kudinova OI, Kovaleva NY, Grinev VG, Ladygina TA, et al. (2009) Effect of the zeolite support on the polymerization of propylene with immobilized ansa-zirconocene catalysts. Polym Sci Ser B 51: 401-408.
- Hlatky GG (2000) Single-site catalysts for olefin polymerization: Annual review for 1997. Coord Chem Rev 199(1): 235-329.
- Severn JR, Chadwick JC, Duchateau R, Friederichs N (2005) “Bound but not gagged” immobilizing single-site α-olefin polymerization catalysts. Chem Rev 105(11): 4073-4147.
- Wang X, Xu R, Zhu B, Li Y, Ma Y (2016) Synthesis and characterization of functional porous organic polymers as efficient metallocene catalyst supports. N J Chem 40: 8324-8333.
- Yugen Zhang, Siti Nurhanna Riduan (2012) Functional porous organic polymers for heterogeneous catalysis. Chem Soc Rev 41(6): 2083-2094.
- Klapper M, Joe D, Nietzel S, Krumpfer JW, Muellen K (2014) Olefin polymerization with supported catalysts as an exercise in nanotechnology. Chem Mater 26: 802-819.
- Lei J, Li D, Wang H, Zhou G (2011) Porous polyethylene spheres with nanofiber structure from Ziegler-Natta catalyst supported on porous polymer particles. Polymer 52: 602-605.
- Sun L, Hsu CC, Bacon DW (1994) Polymer-Supported Ziegler-Natta Catalysts. 1. A Preliminary Study of Catalyst Synthesis. J Polym Sci Part A Polym Chem 32(11): 2127–2134.
- Sven Nietzel, Daejune Joe, Joseph W Krumpfer, Frank Schellenberger, Abdulhamid A Alsaygh, et al. (2015) Organic nanoparticles as fragmentable support for Ziegler-Natta Catalysts. J Polym Sci Part A: Polym Chem 53(1): 15-22.
- Heurtefeu B, Bouilhac C, Cloutet E, Taton D (2011) Polymer support of “single-site” catalysts for heterogeneous olefin polymerization. Prog Polym Sci 36(1): 89-126.
- Wang X, Li Z, Han X, Han Z, Bai Y (2017) Highly tunable porous organic polymer (POP) supports for metallocene-based ethylene polymerization. Appl Surface Sci 420: 496–503.
- Wang X, Han X, Ren F, Xu R, Bai Y (2018) Porous organic polymers-supported metallocene catalysts for ethylene/1-hexene copolymerization. Catalysts 8(4): 146.
- Wang X, Zhang CL, Liu WX, Zhang PS (2018) Feasibility study on the design and synthesis of functional porous organic polymers with tunable pore structure as metallocene catalyst supports. Polymers 10(9): 944.
- Wang X, Kang W, Gao L, Li G, Chen X, Guo Y (2021) Highly flowable nano TiO2/porous organic polymer (POP) supports for efficient metallocene catalysts. Nanomaterials 11(1): 60.
- Wang X, Kang W, Zhang C, Li G, Zhang P, et al. (2022) Sulfonated porous organic polymer supported Zeigler-Natta polypropylene catalysts with high stereoregularity and broad molecular weight distribution. Microporous Mesoporous Mater 343: 112151.
- Zahra-Alsadat Hejazi-Dehaghani, Hassan Arabi, Daniel Thalheim, Danijel Vidakovic, Mehdi Nekoomanesh Haghighi, et al. (2021) Organic versus inorganic supports for metallocenes: The Influence of rigidity on the homogeneity of the polyolefin microstructure and properties. Macromolecules 54: 2667-2680
- McKenna, Timothy FL Bashir, Muhammad Ahsan (2019) In multimodal polymers with supported catalysts: Design and production by Albunia AR, et al. (Eds.), Springer Nature Switzerland AG 2019, pp. 81-114.
© 2023 Xiong Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






