- Submissions

Full Text
Annals of Chemical Science Research
C–H Functionalization of 2-/3- Aroylbenzofurans: A Tool for Developing New Anti-Arrhythmic Drugs
Srinivas Kolluru*
Acme Biosciences (A Frontage Laboratories), Palo Alto, USA
*Corresponding author:Srinivas Kolluru, Acme Biosciences (A Frontage Laboratories), Palo Alto, California, USA
Submission: November 17, 2022;Published: November 28, 2022

Volume3 Issue3November , 2022
Abstract
Aroyl-benzofurans are important heterocyclic compounds which displayed a wide range of biological activities and important structural motif of many pharmaceutical drugs. Most of the pharmaceutical drugs containing the aroyl-benzofuran structural core have severe side effects which limits its usage. Some of the drugs containing the aroyl-benzofuran structural core were withdrawn from the market due to its toxicity. So, there is a need for the development of new synthetic methods to functionalize these molecules which play a significant role in drug discovery program. The current review focuses on the C-H functionalization of 2-/3- aroyl-benzofurans which includes alkylation, arylation, phosphonylation and benzoylation which plays a crucial role in developing new anti-arrhythmic drugs.
Introduction
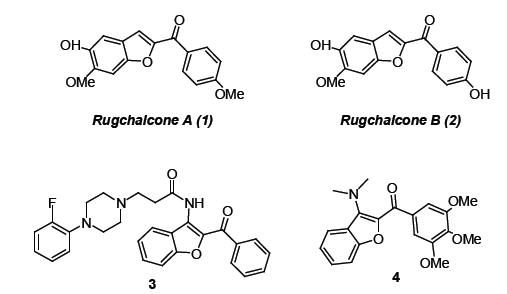
Benzofuran is a heterocyclic compound in which benzene and furan are fused together. The compounds containing the benzofuran skeleton has diverse biological activities such as antifungal [1], anti-microbial [2], anticancer [3,4], calcium entry blockers [5] and many other biological functions. Aroyl-benzofurans are important class of benzofuran derivatives which constitutes the structural moiety of natural products and pharmaceutical drugs. For instance, Rugchalcones A and B contain the 2-aroylbenzofurans structural motif and exhibit the anti-tobacco mosaic virus and anti-inflammatory activities [6]. Compound 3 exhibits the anticonvulsant and neurotoxic activity [7] whereas the compound 4 exhibits inhibition in tubulin polymerization [8] (Figure 1).
Figure 1:Bioactive aroyl-benzofurans.
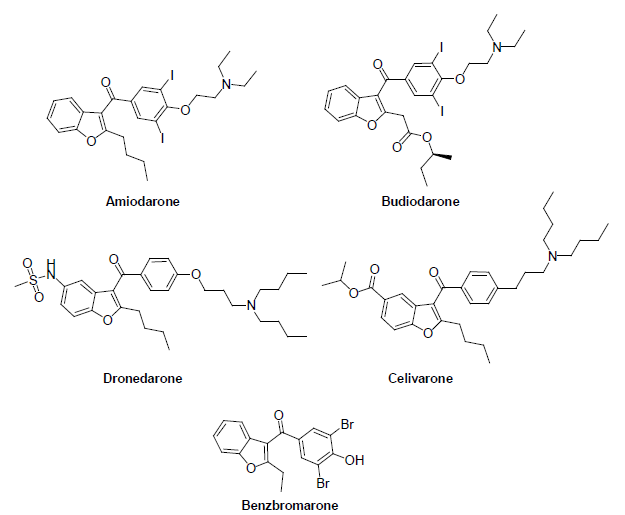
Apart from the above molecules, several pharmaceutical drugs contain the aroyl benzofuran structural core. For instance, Benzbromarone is a 3-aroylbenzofuran derivative< which acts as a uricosuric agent [9]. However, after the serious side efforts of hepatotoxicity it was withdrawn from the market. Amiodarone, Budiodarone, Dronedarone and Celivarone are the 3-aroylbenzofuran derivative which acts as antiarrhythmic drugs (Figure 2) [10]. Amiodarone has the side efforts on thyroid, pulmonary and hepatic toxicity. As a result of this Dronedarone, a structural analogue of Amiodarone was approved as a class III antiarryhthmic drug. Dronedarone does not contain iodine in its structure and as a result the toxicity of this drug on the thyroid was reduced. But rare cases of liver damage were reported with Dronedarone. This reveals the significance of the aroyl benzofurans in medicinal chemistry and also the need of strategies to functionalize these molecules. C-H activation is a powerful tool to functionalize these molecules which play a significant role in drug discovery programs. The current review is focused on the advances in the C-H functionalization of 2/3-aroylbenzofurans.
Figure 2: Pharmaceutical drugs containing the aroyl-benzofuran structural moiety.
C-H alkylation of 2-Aroyl benzofurans
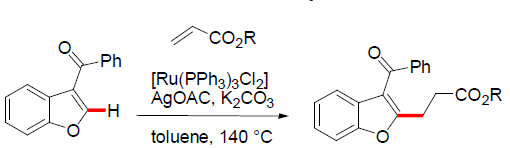
Ramana and coworkers reported for the first-time alkylation reaction on 2-Aroylbenzofurans. The alkyaltion of 2-aroylbenzofurans was explored with acrylate and it occurs selectively in branched alkylation by employing Ru(PPh3)3Cl2 catalyst and linear alkylation with [Ru(p-cymene)Cl2] [2]. The differences in the mode of alkylation were explained in terms of sterics and electronics effects of the catalyst. The presence of bulky PPh3 ligands around the Ru centre in Ru(PPh3)3Cl2 favours the β carbon of acrylate close to the Ru centre in ruthenacycle whereas sterically less hindered [Ru(p-cymene)Cl2]2 the electrophilic β carbon of acrylate is away from the metal centre resulting in the alkylation products. The reaction was explored with numerous acrylates resulting in the corresponding branched and linear alkylation products. Apart from acrylates several other coupling partners were explored in the alkylation reaction and it turns out that styrene, isopropyl acrylamide and dodecene were compatible resulting the corresponding alkylation products whereas acrylonitrile and methylvinyl ketone failed to give the corresponding alkylation products [11-13] (Figure 3).
Figure 3:

C-H alkylation of 3-Aroyl benzofurans
Dr. Ramana and coworkers [12,13] continued their efforts to explore the factors favoring the unusual, branched selectivity in the 2-Aroylbenzofurans. DFT calculations were performed on two operations in the mechanistic cycle –the approach of acrylate to the Ru-C bond of ruthenacycle and insertion of acrylate into the Ru-C bond of ruthenacycle. From this calculation it was concluded that the interplay of the steric vs electronic factors around the ruthenium centre decides the mode of alkylation in 2-Aroylbenzofurans. Further the DFT calculations predict the linear alkylation in 3-Aroylbenzofurans which was verified experimentally. The alkylation of 3-aroylbenzofurans was explored with numerous acrylates to give the linear alkylation products in good to excellent yield. Apart from the benzofuran, the 3-aroylfuran and 2-aroylfurans also gave the mixture of linear and branched alkylation products. The 3-aroylindoles and 3-aryolthiophenes are not compatible in the current transformation [15,16] (Figure 4).
Figure 4:
C-H arylation of 2-Aroyl benzofurans
The arylation of 2-aroylbenzofurans was first investigated by Dr. Emmanuel and coworkers [14]. Aryl bromides were used as coupling partners. The 2-aroylbenzofurans reacted with aryl bromides in presence of Pd(OAC)2 catalyst, P(t-Bu)2Me·HBF4 phosphine ligand, potassium carbonate and PivOH additive gave the C3 arylation products in good to excellent yields. The reaction was explored with various electron donating and electron withdrawing substituents on aryl bromides. Further the reaction was explored with poly-substituted 2-aroylbenzofurans to give the corresponding 2-aroyl-3-(hetero)aryl-benzofurans. Apart from the aryl bromides, the pyridinyl bromides were also explored successfully in the C3 arylation of 2-aroylbenzofurans [14] (Figure 5).
Figure 5:
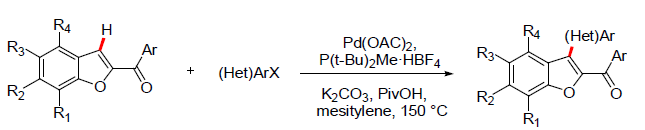
Dr. Ramana and coworkers [13] reported a ruthenium catalyzed C-H (hetero)arylation of 2-Aroyl-benzofurans. In this strategy the authors employed (hetero) aryl-boronic acid and (hetero)aryl- BF3K salts as coupling agents. The reaction condition include treating 2-aroylbenzofuran with aryl-boronic acid in toluene or aryl-BF3K salts in DCE in presence of RuCl2(PPh3)3 and K2CO3 at 140 °C gave the 2-aroyl-3-(hetero) aryl-benzofurans in good to excellent yields. The proposed mechanism include carbonatoruthenium (II) complex as active catalyst in the catalytic cycle. Further from the control experiments the authors proposed the aerobic oxidation of Ru(0) to carbonatoruthenium (II) complex to continue the catalytic cycle [19] (Figure 6).
Figure 6:

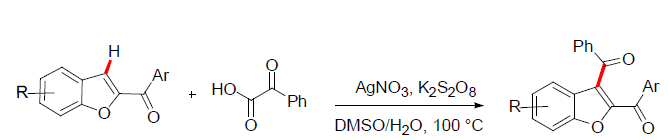
Benzoylation of 2-Aroyl benzofurans
Dr. Pranjal and coworkers [17] explored the C3 aroylation of 2-Aroylbenzofurans. In this approach 2-Aroylbenzofurans was reacted with phenyl glyoxylic acids in presence of silver catalyst to give the 2,3-diaroylbenzofurans. The reaction was explored with variously substituted 2-Aroylbenzofurans to give the corresponding 2,3-diaroylbenzofurans in good yields (Figure 7).
Figure 7:
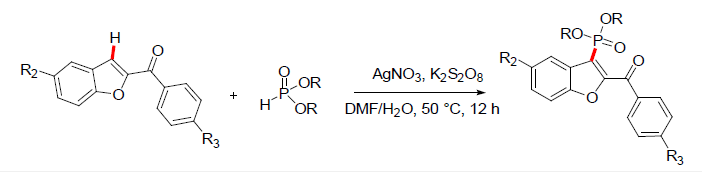
C-H phosphonylation of 2-Aroyl benzofurans
Dr. Kashanna and coworkers [18] have reported the C3 functionalization of 2-aroylbenzofurans by introduction of a phosphorous moiety by dehydrogenative cross coupling reaction. In this approach the 2-aroylbenzfurans were reacted with phosphites in presence of AgNO3 and K2S2O8 to give the 2-aroyl-3- phosphonylbenzofurans. The reaction was explored with a wide variety of substituted 2-aroylbenzofurans and various phosphites resulting in the library of 2-aroyl-3-phosphonylbenzofurans. The aroyl benzofurans with EWG substituents gave lower yields. The reaction was compatible with various dialkylphosphites and diarylphosphites [18] (Figure 8).
Figure 8:
Conclusion
Aroyl-benzofurans are important structural core in antiarrhythmic drugs. Most of the drugs have high levels of toxicity which limits its usage. There is an urgent need for the development of new strategies to functionalize these molecules which plays a crucial role in drug discovery program. C-H activation is a powerful tool to functionalize C-H bonds with various coupling partners. Here we have documented the various reports on C-H functionalization of 2-/3- aroyl-benzofurans which include the alkylation (branched & linear), arylation, phosphonylation and benzyolation which play a significant role in developing new anti-arrhythmic drugs.
<References
- Aslam SN, Stevenson PC, Phythian SJ, Veitch NC, Hall DR (2006) Synthesis of cicerfuran, an antifungal benzofuran, and some related analogues. Tetrahedron 62(17): 4214-4226.
- Wahab Khan M, Jahangir Alam M, Rashid MA, Chowdhury R (2005) A new structural alternative in benzo[b]furans for antimicrobial activity. Bioorg Med Chem 13(16): 4796-4805.
- Shaziyaparveen KS, Vinodh JSS, Srinivas K, Ganesh NP, Santhosh Kumar TR, et al. (2020) Discovery of 3-(benzofuran-2-ylmethyl)-1H-indole derivatives as potential autophagy inducers in cervical cancer cells. Bioorg Med Chem Lett 30(19): 127431–127439.
- Srinivas K, Ramana CV (2017) Total synthesis of propolisbenzofuran B. Org Lett 19(24): 6466-6469.
- Alan P Kozikowski, Dawei Ma, Linh Du, Nancy E Lewin, Peter M Blumberg (1995) Effect of alteration of the heterocyclic nucleus of ILV on its isoform selectivity for PKC. Palladium-catalyzed route to benzofuran analogs of ILV. Farmaco 50(6): 425-430.
- Young Hwa Seo, Kongara Damodar, Jin-Kyung Kim, Jong-Gab Jun (2016) Synthesis and biological evaluation of 2-aroylbenzofurans, rugchalcones A, B and their derivatives as potent anti-inflammatory agents. Bioorg Med Chem Lett 26(6): 1521–1524.
- Mehnaz Kamal, Ashok K Shakya, Mohamed Jawed Ahsan, Talha Jawaid (2013) Synthesis, Anticonvulsant and Neurotoxicity Evaluation of Some Newer N-(2-benzoylbenzofuran-3-yl)-3-(substituted)-propanamide Analogs. Cent Nerv Syst Agents Med Chem 13(3): 159-165.
- Romeo Romagnoli, Pier Giovanni Baraldi, Taradas Sarkar, Maria Dora Carrion, Olga Cruz-Lopez, et al. (2008) Synthesis and biological evaluation of 2-(3′,4′,5′-trimethoxybenzoyl)-3-N,N-dimethylamino benzo[b]furan derivatives as inhibitors of tubulin polymerization. Bioorg Med Chem Lett 16(18): 8419-8426.
- Tomoyuki Ohea, Ryutaro Umezawaa, Yumina Kitagawaraa, Daisuke Yasudaa, Kyoko Takahashia, et al. (2018) Synthesis of novel benzbromarone derivatives designed to avoid metabolic activation. Bioorg Med Chem 28(23-24): 3708-3711.
- Pamela K Mason, John P DiMarco (2009) New pharmacological agents for arrhythmias. Circ Arrhythmia Electrophysiol 2: 588-597.
- Yadagiri Kommagalla, Kolluru Srinivas, Chepuri V Ramana (2014) Ru-Catalyzed branched versus linear selective C3-alkylation of 2-Aroylbenzofurans with acrylates via C–H activation. Chemistry Eur J 20(26): 7884-7889.
- Chepuri Venkata Ramana, Yadagiri Kommagalla, Kolluru Srinivas (2015) A process for the preparation of anti-inflammatory aroylbenzofuran compounds. WO2015029062A1.
- Chepuri Venkata Ramana, Yadagiri Kommagalla, Kolluru Srinivas (2018) Process for the preparation of anti-inflammatory aroylbenzofuran compounds. US9890132B2.
- Amandine Carrër, Dimitri Brinet, Jean-Claude Florent, Patricia Rousselle, Emmanuel Bertounesque (2012) Palladium-Catalyzed direct arylation of poly-substituted benzofurans. J Org Chem 77(3): 1316-1327.
- Kolluru Srinivas, Yuvraj Dangat, Yadagiri K, Vanka Kumar, Chepuri V Ramana (2017) Electronic control on linear versus branched alkylation of 2-/3-aroylbenzofurans with acrylates: Combined DFT and synthetic studies. Chem Eur J 23(31): 7570-7581.
- Kolluru Srinivas, Rashmi Sharma, Chepuri V Ramana (2017) Interrupting base-mediated benzofuran ring transformation with michael acceptors. J Org Chem 82(18): 9816-9823.
- Kashmiri Neog, Babulal Das, Pranjal Gogoi (2018) 2,3-Diaroyl benzofurans from arynes: sequential synthesis of 2-aroyl benzofurans followed by benzoylation. Org Biomol Chem 16: 3138-3150.
- Kashanna Jajula, Rathod Aravind Kumar, Kishore R, Prakash Raj T, Ravula Srikanth, et al. (2022) Silver(I)-catalyzed dehydrogenative cross-coupling of 2-aroylbenzofurans with phosphites. New J Chem 46: 2662-2668.
- Dinesh J Paymode, Chepuri V. Ramana (2015) Ruthenium(II)-Catalyzed C3 arylation of 2-aroylbenzofurans with arylboronic acids/aryl-trifluoroborates via carbonyl directed C–H bond activation. J Org Chem 80(22): 11551-11558.
© 2022 Srinivas Kolluru. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






