- Submissions

Full Text
Annals of Chemical Science Research
BiMeVOxes: From Polycrystalline Electrolytes for SOFCs to Mesoporous Nano-sized Structures as Efficient Photocatalysts
Ahlam Al-Alas and Niyazi AS Al-Areqi*
Department of Chemistry, Faculty of Applied Science, Taiz University, Yemen
*Corresponding author:Niyazi AS Al- Areqi, Department of Chemistry, Faculty of Applied Science, Taiz University, Yemen
Submission: July 18, 2022;Published: September 20, 2022

Volume3 Issue2September , 2022
Abstract
Here, we demonstrated a brief historic review on BiMeVOx materials since 1990 upto now and their functionality was modified from polycrystalline electrolytes for SOFCs to mesoporous nano-sized structures as efficient Photocatalysts for degradation of organic dyes in wastewaters.
Introduction
BiMeVOxes (Bi = bismuth, Me = dopant metal ion, V = vanadium, and Ox = oxide) constitute a family of layered Aurivillius–type compounds, derived by the partial substitution of Me for V in the parent compound, Bi2VO5.5 and formulated as Bi2MexV1-xO5.5-δ. These functional materials were first discovered by Abraham et al. [1] and then extensively investigated by several researchers for their ionic conductivity. The ionic conductivity in Bi2VO5.5 and their derivates, BIMEVOXes is entirely attributed to the existence of such vacancies in the perovskite vanadate layers which thereby facilitate the mobility of oxide ion through [2,3]. The vacancy ordering in the perovskite vanadate layer is associated with the occurrence of two-phase transitions; monoclinic-α to orthorhombic-β at 447 °C and β to tetragonal-γ at 567 °C. Many studies conferred the stabilized γ-BiMeVOx phase a promising application as a polycrystalline solid electrolyte for intermediate temperature-solid oxide fuel cells (IT- SOFCs) (Table 1, Figure1).
Figure 1:Plots of σ600 and high-teperature activation energy (ΔEHT) for BIALVOX system vs. composition.
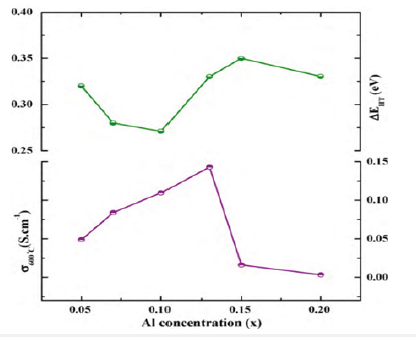
Table 1:Values of electrical conductivity for some stabilized -BiMeVOxes at 300 and 600 ˚C.
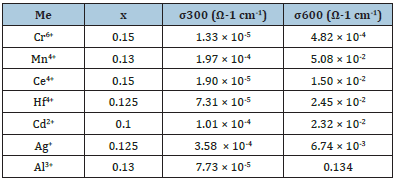
More recently, many investigations made by our research group and others on the electrical properties of BiMeVOxes, however proved that all their stabilized phases behave as semiconductors at temperatures less than 300 °C and have band-gap energies (Eg) in the visible-light region. Therefore, much attention has been paid to finding an alternative application of such materials such as a semiconductor-mediated photo-catalysis for photodegradation of organic pollutants in wastewaters. The first attempt to employ such a type of materials as visible-light photocatalysts was made by Thakral and Uma [4], followed by our study in 2014. As a result of polycrystallinity, these were found to be moderate for photodegradation of organic dyes and showed similar photocatalytic efficiencies.
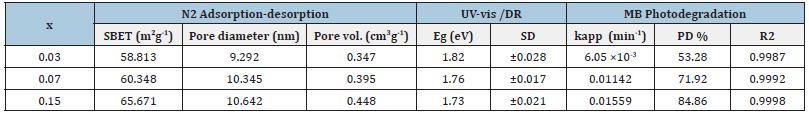
However, our research group in 2022 have successfully developed three mesoporous nano-sized phases (α-monoclinic, β-orthorhombic and γ-tetragonal) of BiFeVOx by means of ethylene glycol-citrate sol-gel synthesis, followed by microwave-assisted calcination and carefully investigated the correlation of the enhanced photocatalytic efficiencies with the phase stability and nanostructure porosity (Figure 2, Table 2 & 3).
Figure 2:Plots of (αhv)2 vs. hv for BiNiVOX.x photocatalysts.
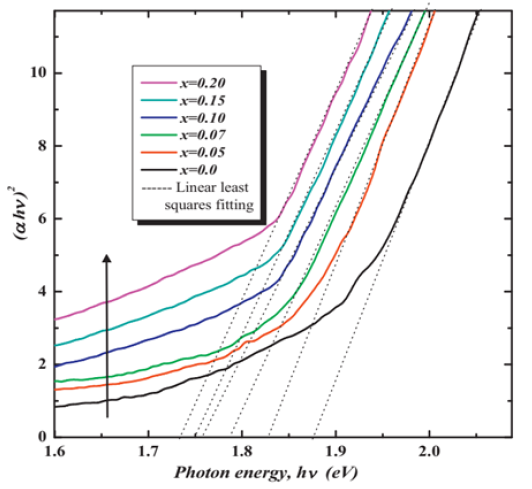
Table 2:Values of Eg for some BiMeVOxes obtained by a simple solid synthesis.
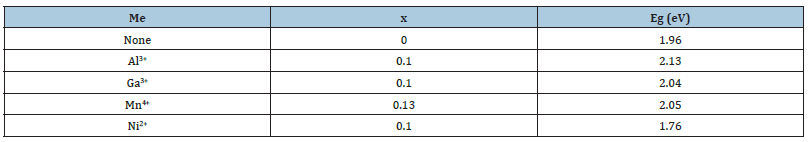
Table 3:Specific surface characteristics, optical properties and methylene blue photocatalytic efficiencies of BiFeVOx photocatalyst series.
<
Conclusion
The highly pronounced photocatalytic efficiency of mesoporous tetragonal γ-BiFeVOx.15 nanophase indicates that the photocatalytic performance of BiMeVOxes with a layered perovskite structure can be further improved by the doping strategy [5,6]. Therefore, the combination of doping strategy with such facile synthesis method plays an important role in improving the structure and surface properties of BiFeVOx. phases and, thereby, enhancing their adsorption and photocatalytic efficiencies.
References
- Abraham F, Boivin JC, Mairesse G, Nowogrocki G (1990) The BIMEVOX series: a new family of high performances oxide ion conductors. Solid State Ionics 40-41: 934-937.
- Beg S, Al-Areqi NAS (2009) Structural and electrical study of CeIV-substituted bismuth vanadate. Journal of Physics and Chemistry of Solids 70(6): 1000-1007.
- Al-Areqi NAS, Beg S, Al-Alas A (2012) Study on phase stability and oxide ion conductivity in the BIAGVOX system. Journal of Physics Chemistry of Solids 73(6): 730-734.
- Thakral V, Uma S (2010) Investigation of visible light photocatalytic behavior of Bi4V2O11-δ and BIMEVOX (ME= Al, Ga) oxides. Materials Research Bulletin 45(9): 1250-1254.
- Al-Areqi NAS, Ahlam Al-Alas, Al-Kamali ASN, Ghaleb KhAS, Al-Mureish K (2014) Photodegradation of 4-SPPN dye catalyzed by Ni(II)-substituted Bi2VO5 system under visible light irradiation: Influence of phase stability and perovskite vanadate-oxygen vacancies of photocatalyst. Journal of Molecular Catalysis A: Chemical 381: 1-8.
- Al-Areqi NAS, Umair M, Senan AM, Al-Alas A, Alfaatesh AMA, et al. (2022) Mesoporous Nano-Sized BiFeVOx.y Phases for Removal of Organic Dyes from Wastewaters by Visible Light Photocatalytic Degradation. Nanomaterials 12(8): 1383.
© 2022 Niyazi AS Al-Areqi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






