- Submissions

Full Text
Annals of Chemical Science Research
Inverse Coordination-The Janus Face of Coordination Chemistry
Ionel Haiduc*
Chemistry Department, Babes-Bolyai University, Romania
*Corresponding author: Ionel Haiduc, Chemistry Department, Babes-Bolyai University, Romania
Submission: October 17, 2021;Published: October 27, 2021

Volume2 Issue5October, 2021
Introduction
I would like to draw attention to the novel chemical concept of inverse coordination, which discloses another face of coordination chemistry (practically ignored so far as a distinct field) and deals with the formation of metal complexes in which the donor and acceptor positions are reversed. This concept emerged after R.C. Mulvey used the “inverse crown” paradigm [1], to describe macrocyclic structures in which “the arrangement of Lewis acidic [acceptor] and Lewis basic [donor] sites is opposite to that encountered in conventional crown ether complexes”. The extension of this paradigm resulted in the “inverse coordination” concept [2]. The inverse coordination can be defined as the formation of metal complexes in which the arrangement of the acceptor and donor sites is opposite to that occurring in conventional coordination complexes [3]. The relationship between traditional coordination complexes and inverse coordination complexes could be regarded as a Janus face image, as suggested by Scheme 1.
Scheme 1:A Janus* face relationship between traditional and inverse coordination complexes. *Janus-Roman god of beginnings and transitions, God of change and time (Wikipedia).
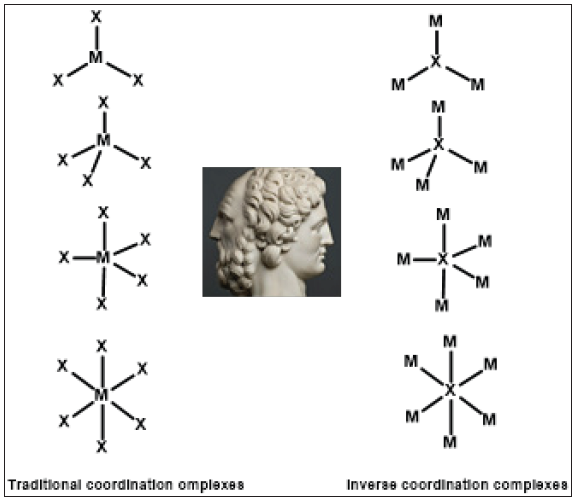
The inverse coordination covers a broad diversity of complexes which can be classified according to the central core, which can be a distinct single atom or a di- or polytopic donor molecule. Several classes of inverse coordination complexes have been reviewed in a book [4] , a general review [5] and several articles, namely dealing with oxygen [3,6], sulfur, selenium [3], halogens [7], nitrogen [8], phosphorus and other pnictogen (arsenic, antimony) [5] single atoms, as well as polytopic exo-donor molecules with oxygen heteroatoms, i.e. oxo-carbons and oxygen heterocycles [9], oxalates and thio and azo analogues [10], nitrogen donor molecules (e.g. five-membered [11] and six-membered [12] nitrogen heterocycles) and inorganic open and cyclic heteroatom molecules [13] as coordination centers. The inverse coordination complexes should be regarded as a distinct chapter within the broad discipline of coordination chemistry.
References
- Mulvey RE (2001) s-Block metal inverse crowns: synthetic and structural synergism in mixed alkali metal–magnesium (or zinc) amide chemistry. Chem Commun, pp. 1049-1056.
- Mulvey RE (2006) “Inverse” Coordination: A New Design Concept in Supramolecular Inorganic Chemistry, EPRSC Grant EP/D076889/1, University of Strathclyde, 2006-2010 and Wright, D. S. EPRSC Grant EP/D077400/1, University of Cambridge, USA.
- Haiduc I (2017) Inverse coordination-An emerging new chemical concept. Oxygen and other chalcogens as coordination centers. Coord Chem Rev 338: 1-26.
- Haiduc I, Tiekink ERT (2020) Inverse coordination chemistry. A novel chemical concept, Sunway University Press, Selangor, Malaysia.
- Haiduc I, Molecules, in press.
- Haiduc I (2021) Inverse coordination complexes. Oxygen as coordination center. In: Constable E, Parkin G, Que L(Eds.), Comprehensive Coordination Chemistry III, 7: 66-120.
- Haiduc I (2017) Inverse coordination-An emerging new chemical concept. II. Halogens as coordination centers. Coord Chem Rev 348: 71-91.
- Haiduc I (2018) Nitrogen centered inverse coordination complexes. A survey of molecular topologies. J Coord Chem 71(9): 3139-3179.
- Haiduc I (2020) Inverse coordination chemistry: oxo-carbons, other poly-oxo carbocyclic molecules and oxygen heterocycles as coordination centers. Topology and systematization. J Coord Chem 73(15): 2117-2170.
- Haiduc I (2020) Inverse coordination metal complexes with oxalate and sulfur, selenium and nitrogen analogues as coordination centers. Topology and systematization. J Coord Chem 73(11): 1619-1700.
- Haiduc I (2019) Inverse coordination. Organic nitrogen heterocycles as coordination centers. A survey of molecular topologies and systematization. Part 1. Five-membered and smaller rings. J Coord Chem 72(13): 2127-2159.
- Haiduc I (2019) Inverse coordination. Organic nitrogen heterocycles as coordination centers. A survey of molecular topologies and systematization. Part 2. Six-membered rings. J Coord Chem 72(17): 2805-2903.
- Haiduc I (2019) Inverse coordination. Inorganic open and cyclic heteroatom molecules as coordination centers. A survey of molecular topologies. J Coord Chem 72(1): 35-52.
© 2021 Ionel Haiduc. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






