- Submissions

Full Text
Annals of Chemical Science Research
Conjugated Pt(II) Poly-Ynes for Opto-Electronic Applications
Rayya A Al Balushi1*, Ashanul Haque2, Thuraya Al Harthy1, Mohammed Al Hinaai1 and Muhammad S Khan3*
1Department of Basic Science, College of Applied and Health Sciences, A’Sharqiyah University, Oman
2Department of Chemistry, College of Science, University of Hail, Kingdom of Saudi Arabia
3Department of Chemistry, Sultan Qaboos University, Oman
*Corresponding author: Rayya A Al Balushi, Department of Basic Science, College of Applied and Health Sciences, A’Sharqiyah University, Oman, Muhammad S Khan, Department of Chemistry, Sultan Qaboos University, Oman
Submission: June 18, 2021;Published: June 28, 2021

Volume2 Issue4June, 2021
Abstract
Conjugated poly(metalla-yne)s have attracted the interest of academic and industrial researchers working in the field of material chemistry. This review highlights advances made in the field of conjugated Pt(II) based poly-ynes. This include some basic aspects of conjugated Pt(II) poly-ynes; synthetic methodologies, structure-property relationships. Some examples of synthesizing non-platinum metalla-ynes are also included. In the application part, the major focus has been directed on the optoelectronic (OE) properties.
Keywords: Conjugated poly(metalla-ynes); Structure-property relationships; Pt(II) polynes; Optoelectronic applications
Abbreviations: BHJ: Bulk heterojunction; CIE: Commission International de LEclairage; EQE: External quantum efficiency; HOMO: Highest occupied molecular orbital; ISC: Intersystem crossing; LCDs: Liquid crystal displays; LEDs: Light emitting diodes; LLCT: Ligand to ligand charge-transfer; LUMO: Lowest unoccupied molecular orbital; MLCT: Metal to ligand charge-transfer; NLA: Nonlinear absorption; NLO: Nonlinear optics; NMR: Nuclear magnetic resonance; OLEDs: Organic light emitting diodes; OPL: Optical power limiters; PCBM: [6,6]-phenyl-C61-butyric acid methyl ester; PC61BM: [6,6]-phenyl-C61-butyric acid methyl ester; PC71BM: [6,6]-phenyl-C71-butyric acid methyl ester; PCE: Power conversion efficiency PLED: Polymer light-emitting diodes; PMMA: Polymethyl methacrylate; PSCs: Polymer solar cells; RSA: Reverse saturated absorption; SCs: Solar cells; SOC: Spin-orbit coupling; TA: Transient absorption; TPA: Two photon absorption; WOLEDs: White organic light-emitting diodes
Introduction
The continuing development in the area of conjugated poly(metalla-yne)s during the last few decades is the result of interdisciplinary research interests of chemists, physicists, materials scientists and engineers [1].This class of materials provides a good opportunity for coupling the chemical, electronic and optical properties of the metal complexes to those of the organic moiety [2]. Conjugated poly(metalla-yne)s are of considerable interest to academic and industrial researchers because of their potential application in electronics and photonics. The synthetic flexibility, ease of processing, and the possibility of tailoring properties to accomplish a desired function makes them attractive candidates for diverse applications in materials science. Today, they find applications in Light Emitting Diodes (LEDs), Liquid Crystal Displays (LCDs) [3], sensors [4], Nonlinear Optical (NLO) materials [5], optical switches [6], optical data storage devices, signal processing [7] and photovoltaic devices (PVs) [8]. The high demand for poly(metalla-yne)s, especially in Opto-Electronic (OE) devices is due to their versatile nature, cost-effectiveness and easy processability compared to conventional inorganic semiconductors, which have high costs and labor-intensive fabrication procedures [9]. Figure 1 depicts a general chemical formula of poly(metalla-yne) backbone. Poly-ynes consist of a varying number of C≡C units, a spacer group R, and auxiliary ligands around metal ions, e.g. trialkyl or triaryl phosphines. The overall physicochemical properties of the conjugated materials depend on the number of triple bonds, spacer groups, heavy metals and phosphine ligands [10]. Many macroscopic properties of the materials can be tuned by varying these components of a polymer at the microscopic level [11].
Figure 1: General chemical formula of (A) an octahedral and (B) a square planar poly(metalla-yne) backbone. R = aromatic and/or heteroaromatic spacers, M = group 6-9 metal ions for (A) and 10-12 metal ions for (B), L = auxiliary ligands and n = Degree of polymerization.
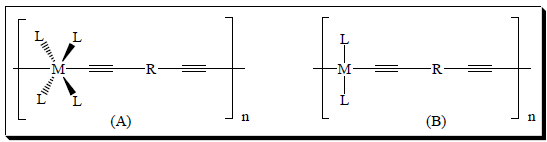
Now it is an established fact that the aforementioned properties of conjugated poly-ynes are structure-dependent, i.e. the properties of the materials can be tuned by varying the chemical structure. For example, conjugated organic poly-ynes are known to show low fluorescent emission (maximum up to 25%) [12] and lack a “spinflip” mechanism. When a heavy metal atom is incorporated into the backbone, it enhances the luminescence properties of the host materials by a process known as “Spin-Orbit Coupling” (SOC) [13]. The resulting SOC allows an efficient ISC and consequently leads to a huge yield of T-excitons (theoretically 100 %) [14]. The extent of SOC and the colour of the luminescence varies from metal to metal. It has also been found that the incorporation of these metal centers and their associated phosphine ligands enhance the solubility of the organic poly-ynes [15].
Among metal containing poly-ynes, Pt(II) poly-ynes are the most studied poly(metalla-yne)s. These complexes have attractive chemical and photophysical properties such as high stability, emission in the visible region, high emission quantum yields and long excited state lifetimes. Over the past few decades, comprehensive research has been conducted on conjugated poly(metalla-yne) materials [16]. This has led to the discovery of several new functional materials with fascinating properties and applications. This review paper describes the design and synthesis of conjugated poly(metalla-yne)s, structure-property relationships and applications.
Discussion
Synthesis of metalla- di-, Oligo- and poly-ynes
Figure 2:Synthetic protocol for poly(metalla-yne)s.
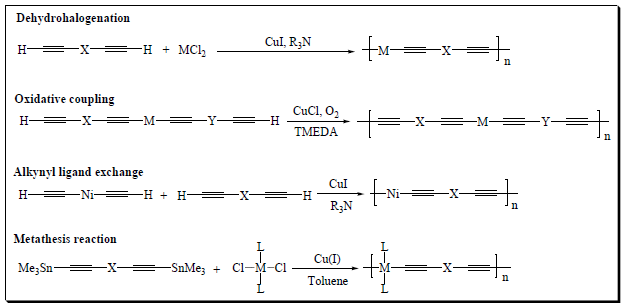
The accidental discovery of the synthetic methodology of ethynyl compounds was reported by C. Glaser in 1869 [17]. He discovered the homo-coupled product of terminal alkynes. Since then the synthesis of oligo-ynes or poly-ynes with precisely defined length, and chemical composition has attracted the attention of polymer chemists. For the synthesis of pre-defined C≡C and C‒M bonds, several cross-coupling reactions between organometallic reagents and organic halides/other electrophiles have been employed. Classically, to introduce ethynyl moieties as substituents onto an aromatic ring and to synthesize a polymeric material, a sequence of Sonogashira, Stille, and Hagihara type metal-catalyzed coupling reactions are commonly used [18,19]. The following sub-sections discuss some of the important classical and recent methodologies employed for the synthesis of conjugated metal-containing compounds, from di-ynes through oligo-ynes to poly-ynes. Metalla-diynes, oligo-ynes and poly-ynes are generally synthesized by using a variety of reactions including dehydrohalogenation condensation reactions, oxidative coupling, ethynyl ligand exchange reactions, transmetallation/metathesis reactions, C-H activation and vinyledene methods (Figure 2). The oxidative coupling reaction is generally employed for the synthesis of terminal alkynes, while the alkynyl ligand exchange reaction is particularly used for Ni-containing poly-ynes. All these methods have their merits and demerits, which have been comprehensively reviewed [16,20].
Takahashi S and co-workers [21,22] first reported the synthesis of soluble group 10 poly(metalla-yne)s using dehydrohalogenation reaction. However, use of an amine solvent was a major drawback of this method as the metal phosphine precursor complexes other than those of the group 10 elements are unstable in amine. To overcome this limitation, inspired by the work of Lappert [23]. Davies SJ et al. [24] developed a more general method for the synthesis of poly(metalla-yne) s (Figure 3). This method has the ability to produce poly-ynes of higher molecular weight than that previously reported by Takahashi S et al [22]. In this method bistrimethylstannyl( ethynyl) (L) offered an excellent precursor for synthesizing metalla-diynes, oligo-ynes and poly-ynes in high yields. When trans-M(PnBu3)2Cl2 (M=Ni, Pd, Pt) was treated with 1.0 equiv. of (L), the metal poly-yne (A) was produced in comparable yields. Similarly, treatment of 2.0 equivs. of (L) with 1.0 equiv. of Ptprecursor trans-Pt(PnBu3)2Cl2 afforded the product (B) having one Pt atom ligated by two trans-acetylide groups and trimethylstannyl acetylide moieties. An in-situ reaction of (B) with excess of trans- Pt(PnBu3)2Cl2 gave (C), with three Pt atoms linked together by two di-acetylide units.
Furthermore, the versatility of the trimethylstannyl(ethynyl)s allowed facile synthesis of poly(metalla-yne)s incorporating metals of group 9. When tetrakis(trimethyl-phosphine) rhodium(I) chloride Ph(PMe3)4Cl was treated with 1.0 equiv. of trimethyl(phenylethynyl) stannane, it produced (D); however, in the presence of excess of trimethyl(phenyl-ethynyl)stannane, a new complex mertrans-[ Rh(PMe3)3(SnMe3)(C≡CPh)2] (E) was reported in good yield. Likewise, changing the stoichiometry and reactants, formation of other species like (F) and (G) were also reported (Figure 3). The main attractive features of this method were its generality and versatility over the other methods. It can be used to produce both the metalla di-ynes and poly(metalla-yne)s of Ni, Pd and Pt having high M.W., good yields (>96%) and purity. However, the neurotoxic nature of the trimethyl tin chloride (Me3SnCl) reagent restricted the use of this method [25].
Figure 3: Synthesis of Pt(II) and Rh(I) complexes.

Fyfe HB et al. [26] reported a high-yield C-H activation method for preparation of dinuclear and oligomeric Rh complexes linked to conjugated acetylides (Figure 4). When [Rh(PMe)3Me] was treated with bis-acetylide incorporating varying aromatic spacers in 2:1 stoichiometry in THF, a molecule of methane was lost to yield (a). However, when the stoichiometry of the starting materials were reversed (1:2), mononuclear Rh(III) complexes mer-trans- [Rh(PMe3)3(H)(C≡C‒X‒C≡CH)2] (c) were obtained. When these two reactants were used in 1:1 stoichiometry, a rigid-rod poly-yne (b) was produced. A model complex (d) and its corresponding poly(metalla-yne) (e) were also reported by the treatment of [Rh(PnBu3)4BPH4] with phenylacetylene (2.0 equivs.) and 1,4-diethynylbenzene (1.0 equiv.) in the presence of strong base (MeLi). In this reaction, the only by-products of the polymerization reactions are CH4 and PR3.
Figure 4:Synthesis of Rh(I) di-ynes and poly-ynes via C-H activation method.

Recently, Fyfe HB et al. [27] reported the use of Sonogashiratype cross-coupling reactions in the preparation of a range of Ru buta-1,3-diynyl complexes from a common Ru(C≡CC≡CH) (PPh3)2Cp platform (2) which was obtained from fluoride-induced desilylation of the readily available complex Ru(C≡CC≡CSiMe3) (PPh3)2Cp (1) (Figure 5). This strategy obviates the need to prepare different di-yne ligands for each and every complex, providing rapid access to a range of complexes with various aryl buta-1,3-diynyl ligands. Reaction of (2) with the aryl iodides in diisopropylamine cocatalyzed by a simple Pd(PPh3)4 (5 mol%)/CuI (10 mol%) mixture gave the substituted buta-1,3-diynyl complexes Ru(C≡CC≡CAr) (PPh3)2Cp (3) in moderate yield. These examples illustrate the versatility of the “chemistry-on-complex” strategy; through this approach buta-1,3-diynyl complexes with electron-withdrawing (C6H4CN) (3)a, electro-neutral (C6H4Me) (3)b, electron-donating (C6H4OMe) (3)c, or metal surface contacting (2,3-dihydrobenzo[b] thiophene (DHBT) (3)d; C5H4N (3)e) substituents have been obtained. The process is suitable for the preparation of “simple” buta-1,3-diynyl complexes, i.e., those bearing substituents, which are chemically and functionally rather complex, such as 2,3-dihydrobenzo[b]thiophene (3)d and pyridine (3)e, and more elaborate bis(diynyl) complexes such as (5).
Figure 5:Sonogashira type reaction for the synthesis of Ru-buta-1,3-diynyl complexes.

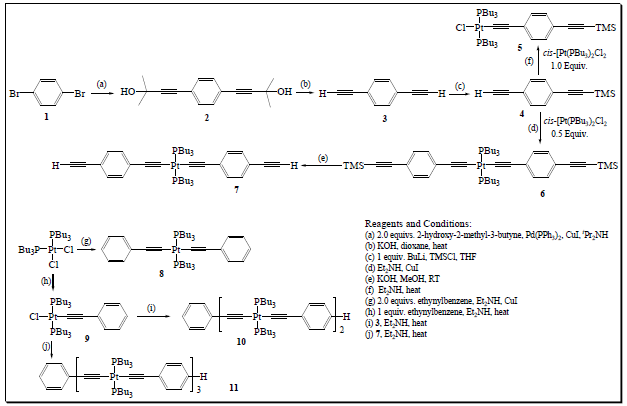
Liu Y et al. [28] reported the synthesis of mono-dispersed Pt(II) oligo-ynes of precise length using an iterative convergent approach (Figure 6). The method uses Pt(II)-ethynyl units as building blocks in which terminal acetylenes were protected using the TMS protecting group. Figure 6 depicts the conversion of 1,4-dibromobenzene (1) to 1,4-diethynylbenzene (4) via a diol compound (2). Selctive conversion of 3 → 4 was achieved at -78 °C using THF/BuLi/TMSCl system. Refluxing 4 with 1.0 and 0.5 equiv. of cis-dichlorobis(tri-nbutylphosphine) Pt(II) salt afforded 5 and 6, respectively. Similiarly, oligo-ynes having different number of Pt(II) ion (8-12) were also obtained by reacting these intermediate compounds in different ratios and conditions.
Figure 6:Synthesis of Pt(II) oligo-ynes.
Structure-property relationships
High molecular weight conjugated poly(metalla-yne)s are often sparingly soluble in organic solvents, thus hindering complete characterization and property determination. The low solubility of the poly-ynes is attributed to the polarizable π electrons, which creates strong intermolecular interactions between the polymeric chains. To alleviate this problem, model compounds can be synthesized along with their polymeric counterparts. Model compounds are building blocks, which can be studied to extrapolate and predict the chemical and photophysical properties of the polymers [29] due to their favourable physical properties like solubility and crystallinity over polymeric materials. On a fundamental level, model compounds are unique in that they provide simplest possible π-conjugated organic units. Such systems are particularly amenable to experimental and theoretical studies focused on issues related to charge and exciton structure and delocalization in linear π-conjugated organic systems. The oligomers of poly-ynes play an important role as model compounds as well as potential materials for device application [30]. Furthermore, the electronic properties of the polymers are influenced by “interchain interactions” in the solid state, so the study of these interactions in the crystal structure of model compounds may lead to a better understanding of interactions in the polymer [31].
Poly(metalla-yne)s have diverse range of applications and possess the capability to resolve many hidden facets at supramolecular level [32]. During the last few decades, research on poly(metalla-yne)s achieved a good momentum because of property modulating nature of metal center and the application of resulting molecules [19,33-35]. Compared to organic polyynes, poly(metalla-yne)s have greater number of components which can be changed to tailor their properties. Organometallic polymers of formula [-M(L)n-C≡CR-C≡C-]∞ can be modified by changing the metal (M), auxiliary ligand (L) or spacer groups (R). A comprehensive literature survey reveals that a wide range of carbocyclic, heterocyclic and main-group element-bridged carbocycles have been used as spacers in the Pt(II) poly-ynes by various research groups worldwide [16,36-39]. The effect that these spacers have on the Pt(II) poly-ynes have been discussed in terms of enhanced conjugation, fused rings, conjugation interruption, ring functionalization and D-A structural motif [36].
Applications of poly(metalla-yne)s
In the following subsections, some recent progress on the application of poly(metalla-yne)s in PVs, NLOs, and LEDs are highlighted.
Photovoltaics (PVs)
With the exponential growth of population, the energy demand also mounted along with other daily needs. Among them, energy is, of course, at the top. Today, for getting energy we are mostly dependent on the non-renewable resources, which are expected to end by the middle of this century. No doubt, this is also one of the culprits of tension among several nations. Every day, the public are feeling the importance of an alternative energy source. Among different alternatives, sun is the tremendous source of energy, and the solar lights can be utilized to replace the non-renewable sources. This is an effective and green approach to get energy. The conversion of solar light into electrical energy can be achieved using Solar Cells (SCs), which are made up of inorganic, organic or polymeric materials [34]. SC devices are comprised of different components, which govern the overall performance of the cells. These include electrodes, interfacial layer, and active materials composed of donor (D) and acceptor (A). All these components contribute to the overall efficiency of a SC. However, most of the research has been dedicated to the development of novel donor materials. In the past two or three decades there has been a large volume of research relating to the development of donor materials [40,41], particularly conjugated poly-ynes and poly(metalla-yne) s incorporating a variety of spacer groups because of their good absorption profile, energy conversion ability, processability and low-cost [42,43]. Conjugated polymers and their use in polymer PVs offer a great technological potential as renewable energy sources for electrical energy and they are currently one of the most promising approaches for the next generation thin-film photovoltaic devices [44-46].
Despite several advantages of conjugated polymers, the major challenge which still persists is to optimize the efficiency and Eg of the PVs based on these materials so that maximum efficiency can be achieved in the terrestrial solar spectrum [47,48]. The efficiency of a polymer solar cell depends upon various factors like Eg, nature of copolymerization and T-excitons. It has been reported that a Eg of 1.3 -1.5 eV is regarded as ideal for polymer-fullerene Bulk Heterojunction (BHJ) solar cells [49]. To narrow the Eg of the polymeric materials and to improve Power Conversion Efficiency (PCE), several modifications have been made so far [16,36,32]. For example, introduction of metal ion showed a synergistic effect on the PCE of solar cells by fine-tuning of HOMO-LUMO Eg through the interaction of the metal d-orbitals with the ligand orbitals. Metal ions also provide redox-active and paramagnetic centers to generate active species for charge transport. Pt(II) acetylide chromophores typically display ground state absorption in the UV region (λ < 420nm) and they are relatively transparent throughout the visible region. Nevertheless, by incorporating segments of alternating D-A arylene units, low Eg Pt(II) acetylide polymers can be obtained featuring strong absorption in the visible region for PV applications. Variable PCEs have been found in the PVs based on blends of Pt(II) poly-ynes incorporating benzothiadiazole-thienyl (12, Figure 7) and benzothiadiazole-3,4-ethylenedioxy thiophene (13, Figure 7) as spacers [50,51]. Mei J et al. [52] reported the enhanced PV performance of 12 and 13 (overall PCEs between 1.1 - 1.4 %). However, Wong et al. [51] reported a remarkably higher efficiency (4.93 %) of the device based on polymer incorporating benzothiadiazole-thienyl spacer [51]. Furthermore, when thienylene spacer flanked by 3,4-ethylenedioxythiophene moieties (13, Figure 7) was blended with methanofullerene, a PCE of 0.30 % was found [53].
Figure 7:Pt(II) di- and poly-ynes with different heterocyclic spacers.
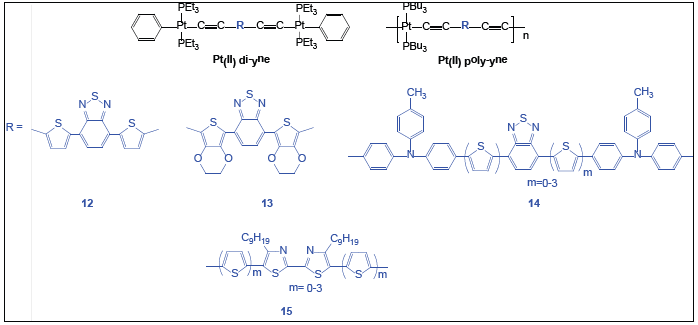
An enhanced light-harvesting ability and solar cell efficiency was reported [54] upon the incorporation of additional thiophene rings in the polymer (14, Figure 7). A PCE of 1.61 % was achieved with Pt(II) poly-yne having two thiophene rings compared to the PCE of 1.09 % for Pt(II) poly-yne without thiophene [36]. Enhancing the absorption coefficient of the band by increasing the polymer conjugation chain length with additional thienyl rings is an effective way to improve the cell performance [54]. Wong’s group developed a series of solution processable Pt(II) polyynes containing bithiazole-oligothienyl rings (15, Figure 7) with strong visible-light absorption [55]. The PCE as well as optical and charge-transport properties of the polymers have been found to be tunable by the number of thienyl rings (m). PCE of the polymer was increased with the lengthening of oligothienyl chain. This work provides an attractive approach to the development of conjugated metallopolymers with broad solar absorption and tunable lightharvesting ability and demonstrated the potential of utilizing metallated conjugated polymers for efficient power generation [36].
Blending of poly-ynes and poly(metalla-yne)s often results in enhanced PCE of the solar cells. An excellent class of strongly visible-absorbing low Eg poly(metalla-yne)s was reported with PCE of over 4% without annealing or the use of tandem structures (12, Figure 7) [56]. Moreover, the solubility profile of the Pt(II) poly-ynes was better than their organic poly(hetero-aryleneethynylene) counterparts. Upon varying the number of thienyl rings, the absorption features and charge carrier mobilities of the resulting metallopolymers were also enhanced. PSCs fabricated with 15 with PCBM as an electron acceptor gave PCE value between 0.21-2.66% at the blend ratio of 1:4 [55].
The solution-processable broad-light absorbing crystalline Pt(II) alkynyl oligomers containing benzothiadiazole central core and different number of thiophenes at termini (16a - c, Figure 8) showed nearly the same Eg value of 1.9 eV and PCE up to 3.0% [43,57]. Inspite of varying number of oligothiophene units, their HOMO level remains on the core unit as indicated by similar open circuit voltage (Voc, 0.71 - 0.82 V). In each case (16a -c, Figure 8), best PCE was achieved using PC71BM. The 1:4 ratio of active layer gives PCE of 1.4 and 2.3% with 16a, 1.7 and 3.0% with 16b and 1.5 and 2.2% with 16c using PC61BM and PC71BM, respectively. The performance of 16b:PCBM, was the best among the series with different electron donors. Similarly, external quantum efficiency (EQE) (50%) was seen in the case of PC71BM than PC61BM (40%) at 440nm. The best result with PC71BM was attributed to the enhanced absorption properties of PC71BM.
Figure 8:Some important Pt(II) poly-ynes as PVs.
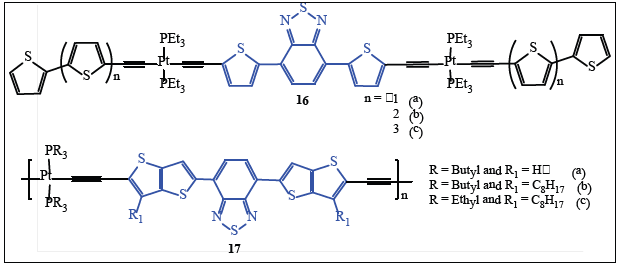
Another amorphous Pt(II) poly-ynes incorporating alternating thiophene (12, Figure 7) or thieno[3,2-b]thiophene (17a - c, Figure 8) connected to 2,1,3-benzothiodiazole showed Eg of 1.81 - 1.85 eV. The device based on polymer:PCBM or PC71BM gave its maximum performance at the thickness of 80 nm and ratio of 1:4. Polymer 17c having lowest Eg gives the maximum Voc (0.79), a short circuit current, Jsc = 10.12 mA/cm2, a fill factor, FF 51.4%, and a PCE of 4.13% [42].
Non Linear Optics (NLOs)
Optical Power Limiters (OPL) are specially designed devices used to filter out radiations of unwanted frequencies. These limiters have found application in various domains of science and technology such as LASER, medicine, sensor, and optical data storage, etc. These devices are made in such a way that their spectral response matches the operating waveband of the device/ sensor/organ, which is intended to protect. OPL possesses the property of nonlinear absorption (NLA), in which the absorption of the light by the material depends on the intensity of waves passing through it. It has been reported that a material may show NLO either through multiphoton absorption (e.g., two- or threephoton absorption) or excited state absorption (also known as reverse saturable absorption) mechanism [58]. High optical transparencies, good optical nonlinearity, excellent solubility, high transmission zone (around 532nm) and a quick response speed are the main pre-requisites for good OPL. These conditions are well fulfilled by organometallic-based materials. Fullerenes (C60), metallo-phthalocyanines, carbon nanotubes, diacetylenes and other organometallic compounds are examples of organometallicbased materials that display NLO behaviour [59]. However, uses of most of these materials are limited by their poor solubility and deep colour, causing difficulties in processing, and hindering the development of practical devices. To curb this drawback, a more soluble and optically transparent material is needed. In this context, researchers are looking positively to organometallic polyynes. Many experimental and theoretical studies supported the fact that the polarizability of ethynyl compounds could be enhanced by extending the conjugation length and the use of D-A groups in the molecule [16,32]. It has now been established that the most versatile Pt(II) poly-ynes demonstrate large hyperpolarizibilities (a unit to compare NLA) compared to purely organic counterparts and this property of Pt(II) poly-ynes is close to inorganic semiconductors. Recently, a theoretical calculation of dipole polarizability (α) and second hyperpolarizability (γ) of the linear CuC2nCu and CuC2nH (n= 1-7) molecules indicated the importance of a metallic end capping on NLO properties. It was found that the cap of Cu largely enhanced the NLO responses for the short chain molecule. The replacement of H by Cu significantly increases the αL and γ L values, especially γL values of short chain poly-ynes (n= 1-3). However, this enhancement of magnitude by Cu rapidly decreases with an increase of chain length (n) and vanished at the effective conjugation length. These behaviours were attributed to the redistribution of charge driven by an external field and the natural bond orbital delocalization.
Figure 9:List of some important poly-ynes as OPLs.
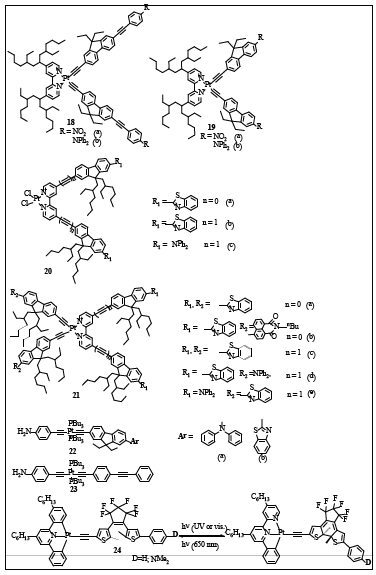
Sun and co-workers found that the photophysical properties of Pt complexes (18a -b and 19a -b, Figure 9) can be tuned drastically by extending the π-conjugation of the acetylide ligands [60]. Transient absorption (TA) study indicated that 19a and 19b exhibit ultrafast ISC and broad band excited-state absoprtion in 450-800nm. Their triplet-excited states appear to be populated via rapid ISC in only a few picoseconds after laser excitation. In addition, strong reverse saturated absorption (RSA) was observed at 532nm for ns laser pulses from 18a and 18b, which are drastically stronger than each of their corresponding complex with shorter π-conjugation in their acetylide ligands i.e. 19a -b. The photophysical and nonlinear transmission studies revealed that extending the π-conjugation of the acetylide ligand can alter the singlet and triplet excited state properties substantially and improve the RSA at 532nm drastically. The broad excited-state absorption and strong nonlinear transmittance performance at 532nm for 18a and 18b suggest that these complexes could be promising candidates as broadband nonlinear absorbing materials.
The same group recently studied the Pt(II) diimine complexes bearing benzothiazolyl fluorenyl, diphenylaminofluorenyl, or naphthalimidylfluorenyl motifs (Figure 9) and its influence on the photophysics of the complexes [61]. The TA spectra showed that both 20a and 20b possess a broad positive absorption bands at∼530nm and a bleaching band at 410 and 420nm, respectively. In comparison to corresponding Zn complexes, Pt complexes possessed much broader positive absorption band and shorter lifetimes, attributed to mixed 3π,π*/3MLCTstates. Likewise, complexes 21a-c exhibit bleaching of the ground-state absorption below 380 nm and narrow positive absorption bands between 450 and 600nm, while 21e shows a bleaching band at 405 nm and a broad positive absorption band between 425 and 750 nm. The TA of complexes 21a - c were reported to emanate from an excited state different from the emitting state and was assigned to 3π,π* states, possibly mixed with minor 3MLCT/3LLCT characters.
Overall, the TA spectra suggest that most of the Pt complexes exhibited stronger triplet excited-state absorption than that of the ground state in the visible spectral region; thus, RSA is anticipated to occur in the visible spectral region for these Pt(II) complexes. Nonlinear transmission experiment for 20 and 21 at 532 nm indicated the occurrence of RSA as the incident energy increases, the transmission of the sample solution decreases. The strength of the RSA at 532nm for these complexes follows the trend: 20b > 21a > 21b ≈ 20a > 21d > 21c > 20c > 21e, with 20b exhibiting the strongest RSA. This trend clearly indicates that incorporation of electron donating substituent NPh2 on the bipyridyl ligand significantly decreases the RSA, as manifested by 20c and 21e. On the other hand, shorter π-conjugation in the bipyridyl ligand increases the RSA at 532 nm, which is evident by the RSA of 21a and 21b in comparison to those of 21c and 21d. Therefore, the substituent on either of the acetylide ligands or the bipyridyl ligand affects the singlet and triplet excited state characteristics significantly, which subsequently influences the RSA.
Most of the OPL measurements are conducted in solution where the nonlinear absorbent is dissolved in a suitable solvent. However, for real life application, solid-state materials are preferred. Pt(II) di-ynes containing 4-(diphenylamino)-fluorene (22a, Figure 9) and 4-(benzothiazole)fluorene (22b, Figure 9) units in PMMA based host material exhibited a moderately large TPA cross-section in the region of 600 -800nm and efficient NLA response to nanosecond pulses via the TPA/ESA mechanism [62]. Shelton AH, et al. [62] studied the effect of incorporation of these highly efficient TPA chromophores 22a and 22b into polymer matrices of Pt(II) di-ynes on their optical and NLA response. The absorption spectra of the monomers, polymers in solution, film, and monoliths were similar and displayed a strong absorption band in the near-UV region for monomers and polymers. At the excitation wavelength of 600 nm, the response trend of the polymeric chromophores was in the order: 23(PMMA) ~ 22b(PMMA) < 22a(PMMA). The comparatively weaker nonlinear absorption response of the 22b(PMMA) and 23(PMMA) materials were attributed to the lower TPA cross sections of 22b and 23 chromophores compared to 22a. [62] In spite of some promising results, the main obstacle in the work was the chromophore loading. A maximum loading of organic guest (~ 10 - 12 %) into the PMMA host was achieved, which was less than that reported earlier using the same host (PMMA) [63]. Vast literatures are available on NLO of metallo-ynes, in both solution and solid states, but no example of reversible metallo-NLO switch in the solid state was available, until the discovery of switchable NLO polymer films based on dithienylethene-based Pt(II) complexes (24, Figure 9) [63]. These compounds undergo photo-induced switching of their second-order nonlinear optical properties. Due to the presence of n-hexyl substituents, these novel Pt(II) complexes were easily poled into polymeric films which also exhibited second-order NLO response and contrast.
Light Emitting Diodes (LEDs)
The working principle of LEDs is just reverse of the solar cells. In light emitting-diodes, electrical excitation occurs at both S and T-excited states. Organic LEDs (OLEDs) are considered as more versatile than conventional inorganic LEDs due to their flexible nature, low cost and energy efficient technology [64]. OLEDs have recently become popular candidates for research into lighting technology, owing to their potential applications in solidstate lighting and flat-panel displays. Additionally, they also offer the distinct advantages of high luminous efficiency, a full-color range, and low manufacturing costs [65,66]. Ethynyl compounds achieved a good reputation in the field of LED technology as both in the role of emitters and dopants to give Electroluminescence (EL) with high brightness and efficiency. A small ISC rate constant and larger fluorescence emission, which are important criteria for White-OLEDs (WOLEDs), have been reported in Pt(II) oligo-ynes incorporating diethynyl benzene spacer (n=1 -3) [67].
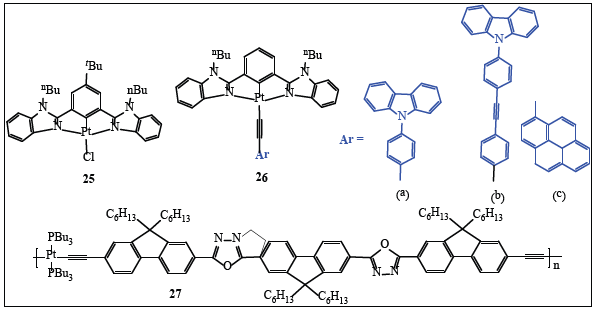
A new class of complexes [Pt(bzimb)Cl] (where bzimb=1,3- bis(N-alkylbenzimidazol-2’-yl)benzene) (25 and 26, Figure 10) with unique photophysical properties have been reported [65]. The functional group present on benzene ring was found to control the emission color of the complexes. Furthermore, the alkynyl moiety in the complex was responsible for an enhanced PL quantum efficiency. Devices composed of dual emissive layer of 25 demonstrated high current efficiencies (40.3 cdA-1) and EQEs (11.8 %). On the other hand, solution-processable devices with complexes 26a and 26b exhibited EQEs of 3.4 % and 1.84 %, respectively. Interestingly, complex 26c possessed green and red emissions attributed to 3IL(bzimb)/3MLCT and 3IL(C≡C─R)/3MLCT excited states. The same complex represented a very first example of white-light EL emission at low concentrations as device with 5% complex 26c gives white-light emission with Commission Internationale de LEclairage (CIE) coordinates of (0.35, 0.39), which were very close to those of pure white light (0.33, 0.33). At dopant concentration of 10% and 20%, the CIE coordinates were (0.37, 0.43) and (0.43, 0.38), respectively.
Pt(II) Poly-yne (27, Figure 10) incorporating alternating oxadiazaole and 9-substituted fluoerene showed HOMO at -6.52 eV and LUMO at around-3.63 eV. This combination of poly-ynes displayed good potential as LED and single-dopant for white PLEDs [67]. A Device doped with 1% of 27 gave the best performance as the maximum external quantum efficiency (ηext) was 0.15%, a luminance efficiency (ηL) of 0.58 cdA-1, and a power efficiency (ηp) of 0.16 lmW-1. A decrease in ηext above 1% was attributed to the concentration quenching effect. To act as a WOLED, the x and y coordinates of CIE chromaticity should be equal to (0.33, 0.33). Here in this case, a device based on 27 was very close to the coordinates and was fairly white and closely approach that of white light using a single dopant configuration.
Figure 10:Some important poly-ynes as LEDs.
Conclusion
Poly(metalla-ynes) have a bright future in opto-electronic industries. Today, we have a good range of synthetic methodologies available for poly(metalla-ynes) of varying length and nature. Even a small variation in the core structure can produce a dramatic change in properties. To understand the photophysical processes occurring at supramolecular level, further improvement is clearly desirable. For example, in-depth knowledge of S and T-excitons is of paramount importance for LED applications. Pt(II) poly-yne of well-defined incorporating varying spacers may be good choice. Similarly, for PV applications, several state-of-the-art new materials comprising Pt(II) and alternating D-A systems have been reported. Considering all these facts, we hope for some new arrivals among this class of materials in the near future, which will show better efficiency than the currently available materials in opto-electronics.
Acknowledgment
RAA gratefully acknowledges the Ministry of Higher Education, Research and Innovation, Oman (Project No. BFP/RGP/EI/18/076) for funding and A’ Sharqiyah University, Oman, for a research grant (ASU-FSFR/CAHS/DBS -01/2019). MSK acknowledges the Ministry of Higher Education, Research and Innovation (MoHERI), Oman for funding (Grant: RC/RG-SCI/CHEM/20/01).
References
- Tour JM (2000) Molecular electronics. Synthesis and testing of components. Acc Chem Res 33(11): 791-804.
- Silvestri F, Marrocchi A (2010) Acetylene-based materials in organic photovoltaics. Int J Mol Sci 11(4): 1471-1508.
- Tang MC, Tsang DPK, Chan MMY, Wong KMC, Yam VWW (2013) Dendritic luminescent gold(III) complexes for highly efficient solution-processable organic light-emitting devices. Angew Chem Int Ed Engl 52(1): 464-469.
- Sun P, Jiang Y, Xie G, Yu J, Du X, et al. (2010) Synthesis and sensitive properties of poly-(bistriethylphosphine)-platinum-diethynylbenzene for organic vapor detection. J Appl Polym Sci 116(1): 562-567.
- Silva TJL, Mendes PJ, Santos AM, Garcia MH, Robalo MP, et al. (2014) Mono(η 5 -cyclopentadienyl)metal(II) complexes with thienyl acetylide chromophores: Synthesis, electrochemical studies, and first hyperpolarizabilities. Organometallics 33(18): 4655-4671.
- Joachim C, Gimzewski JK, Aviram A (2000) Electronics using hybrid-molecular and mono-molecular devices. Nature 408(6812): 541-548.
- Meng F, Hervault YM, Shao Q, Hu B, Norel L et al. (2014) Orthogonally modulated molecular transport junctions for resettable electronic logic gates. Nat Commun 5(1): 1-9.
- Guo F, Kim YG, Reynolds JR, Schanze KS (2006) Platinum-acetylide polymer based solar cells: Involvement of the triplet state for energy conversion. Chem Commun (Camb)17: 1887-1889.
- Bagnis D, Beverina L, Huang H, Silvestri F, Yao Y, et al. (2010) Marked alkyl- vs alkenyl-substitutent effects on squaraine dye solid-state structure, carrier mobility, and bulk-heterojunction solar cell efficiency. J Am Chem Soc 132(12): 4074-4075.
- Lapprand A, Khiri N, Fortin D, Jugé S, Harvey PD (2013) Organometallic oligomers based on bis(arylacetylide)bis(P-chirogenic phosphine)platinum(II) complexes: Synthesis and photonic properties. Inorg Chem 52(5): 2361-2371.
- Kenry, Chen C, Liu B (2019) Enhancing the performance of pure organic room-temperature phosphorescent luminophores. Nat Commun 10(1): 1-15.
- Friend RH, Gymer RW, Holmes AB, Burroughes JH, Marks RN et al. (1999) Electroluminescence in conjugated polymers. Nature 397(6715): 121-128.
- Dray AE, Rachel R, Saxton WO, Lewis J, Khan MS, et al. (1992) Structure of a transition metal-containing polyyne. Macromolecules 25(13): 3473-3479.
- Wittmann HF, Friend RH, Khan MS, Lewis J (1994) Optical spectroscopy of platinum and palladium containing poly‐ynes. J Chem Phys 101(4): 2693-2698.
- Adachi C, Baldo MA, Thompson ME, Forrest SR (2001) Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J Appl Phys 90(10): 5048-5051.
- Haque A, Al-Balushi RA, Al-Busaidi IJ, Khan MS, Raithby PR (2018) Rise of conjugated Poly-ynes and poly(Metalla-ynes): From design through synthesis to structure-property relationships and applications. Chem Rev 118(18): 8474-8597.
- Adimurthy S, Malakar CC, Beifuss U (2009) Influence of bases and ligands on the outcome of the Cu(I)-catalyzed oxidative homocoupling of terminal alkynes to 1,4-disubstituted 1,3-diynes using oxygen as an oxidant. J Org Chem 74(15): 5648-5651.
- Wong WY, Guo YH (2008) Synthesis, structure and photophysics of binuclear gold(I) and mercury(II) complexes derived from 2,5-bis(ethynylphenyl)-1,3,4-oxadiazole. J Mol Struct 890(1-3): 150-156.
- Khan MS, Al-Mandhary MRA, Al-Suti MK, Al-Battashi FR, Al-Saadi S, et al. (2004) Synthesis, characterisation and optical spectroscopy of platinum(II) di-ynes and poly-ynes incorporating condensed aromatic spacers in the backbone. Dalton Trans (15): 2377-2385.
- Bunz UHF (2000) Poly(aryleneethynylene)s: Syntheses, properties, structures, and applications. Chem Rev 100(4): 1605-1644.
- Takahashi S, Kariya M, Yatake T, Sonogashira K, Pittman JCU, et al. (1978) Orgnometallic polymers, Academic Press, New York, USA. Volume 145.
- Takahashi S, Morimoto H, Murata E, Kataoka S, Sonogashira K, et al. (1982) Studies on polyyne polymers containing transition metals in the main chain. VII. Synthesis and characterization of palladium-polyyne polymers. J Polym Sci Polym Chem Ed 20(2): 565-573.
- Cardin CJ, Cardin DJ, Lappert MF (1977) Unsaturated σ-hydrocarbyl transition-metal complexes. Part 2. Synthesis and reactions of vinylplatinum complexes and a comparison with analogous fluorovinyl and alkynyl complexes. J Chem Soc Dalton Trans 8: 767-779.
- Davies SJ, Johnson BFG, Khan MS, Lewis J (1991) Synthesis of monomeric and oligomeric bis(acetylide) complexes of platinum and rhodium. J Chem Soc Chem Commun 3: 187-188.
- Brown AW, Verschoyle RD, Street BW, Aidridge WN, Grindley H (1984) The neurotoxicity of trimethyltin chloride in hamsters, gerbils and marmosets. J Appl Toxicol 4(1): 12-21.
- Fyfe HB, Mlekuz M, Zargarian D, Taylor NJ, Marder TB, et al. (1991) Synthesis of mononuclear, dinuctear and oligomeric rigid-rod acetylide complexes of rhodium, and the molecular structure of [Rh(PMe3)4 (C≡C–p-C6 H4–C≡C)Rh(PMe3)4]. J Chem Soc Chem Commun 3: 188-190.
- Oerthel MC, Yufit DS, Fox MA, Bryce MR, Low PJ (2015) Syntheses and structures of buta-1,3-diynyl complexes from on complex cross-coupling reactions. Organometallics 34(11): 2395-2405.
- Liu Y, Jiang S, Glusac K, Powell DH, Schanze KS, et al. (2002) Photophysics of monodisperse platinum-acetylide oligomers: Delocalization in the singlet and triplet excited states. J Am Chem Soc 124(42): 12412-12413.
- Higari MA, Birckner E, Heise B, Klemm E (1999) Synthesis of some novel heteroarylene ethynylene polymers containing 1, 10‐phenanthroline in the polymer backbone. J Polym Sci Part A Polym Chem 37(23): 4442-4448.
- Mullen K, Wegner G (1998) Electronic materials: The oligomer approach. In Oligomers as Structural Models for Polymers, Wiley-VCH, New York USA, pp. 296-318.
- Cornil J, dos Santos DA, Crispin X, Silbey R, Brédas JL, et al. (1998) Influence of interchain interactions on the absorption and luminescence of conjugated oligomers and polymers: A quantum-chemical characterization. J Am Chem Soc 120(6): 1289-1299.
- Dong H, Zhu H, Meng Q, Gong X, Hu W (2012) Organic photoresponse materials and devices. Chem Soc Rev 41(5): 1754-1808.
- Liu Y, Li Y, Schanze KS (2002) Photophysics of π-conjugated oligomers and polymers that contain transition metal complexes. J Photochem Photobiol C Photochem Rev 3(1): 1-23.
- Gupta A, Watkins SE, Scully AD, Singh TB, Wilson GJ, et al. (2011) Band-gap tuning of pendant polymers for organic light-emitting devices and photovoltaic applications. Synth Met 161(9-10): 856-863.
- Nguyen MH, Nguyen VH, Yip JHK (2013) Sequence-specific synthesis of platinum-conjugated trichromophoric energy cascades of anthracene, tetracene, and pentacene and fluorescent black chromophores. Organometallics 32(24): 7283-7291.
- Ho CL, Yu ZQ, Wong WY (2016) Multifunctional polymetallaynes: Properties, functions and applications. Chem Soc Rev 45(19): 5264-5295.
- Al-Balushi RA, Haque A, Jayapal M, Al-Suti MK, Husband J, et al. (2016) Experimental and theoretical investigation for the level of conjugation in carbazole-based precursors and their mono-, di-, and polynuclear Pt(II) complexes. Inorg Chem 55(13): 6465-6480.
- Al-Balushi RA, Haque A, Jayapal M, Al-Suti MK, Husband J, et al. (2016) Impact of the alkyne substitution pattern and metalation on the photoisomerization of azobenzene-based platinum(II) diynes and polyynes. Inorg Chem 55(21): 10955-10967.
- Al-Busaidi IJ, Haque A, Husband J, Al Rasbi NK, Abou-Zied OK, et al. (2021) Electronic and steric effects of platinum(II) di-yne and poly-yne substituents on the photo-switching behaviour of stilbene: Experimental and theoretical insights. Dalton Trans 50(7): 2555-2569.
- Grätzel M (2009) Recent advances in sensitized mesoscopic solar cells. Acc Chem Res 42(11): 1788-1798.
- Mathew S, Yella A, Gao P, Baker RH, Curchod BFE (2014) Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem 6(3): 242-247.
- He W, Livshits MY, Dickie DA, Yang J, Quinnett R, et al. (2016) A roller-wheel Pt-containing small molecule that outperforms its polymer analogs in organic solar cells. Chem Sci 7(9): 5798-5804.
- Zhao X, Piliego C, Kim B, Poulsen DA, Ma B, et al. (2010) Solution-processable crystalline platinum-acetylide oligomers with broadband absorption for photovoltaic cells. Chem Mater 22(7): 2325-2332.
- Coakley KM, McGehee MD (2004) Conjugated polymer photovoltaic cells. Chem Mater 16(23): 4533-4542.
- Khan MS, Al-Suti MK, Maharaja J, Haque A, Al-Balushi R, et al. (2016) Conjugated poly-ynes and poly(metalla-ynes) incorporating thiophene-based spacers for solar cell (SC) applications. J Organomet Chem 812: 13-33.
- Brabec CJ (2004) Organic photovoltaics: Technology and market. Sol Energy Mater Sol Cells 83(2-3): 273-292.
- Kazmerski LL (2006) Solar photovoltaics R&D at the tipping point: A 2005 technology overview. J Electron Spectros Relat Phenomena 150(2-3): 105-135.
- Ma W, Yang C, Gong X, Lee K, Heeger AJ (2005) Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv Funct Mater 15(10): 1617-1622.
- Scharber MC, Mühlbacher D, Koppe M, Denk P, Waldauf C, et al. (2006) Design rules for donors in bulk-heterojunction solar cells-towards 10 % energy-conversion efficiency. Adv Mater 18(6): 789-794.
- Younus M, Köhler A, Cron S, Chawdhury N, Al‐Mandhary MR, et al. (1998) Synthesis, electrochemistry, and spectroscopy of blue platinum (II) polyynes and diynes. Angew Chemie Int Ed 37(21): 3036-3039.
- Wong WY, Wang XZ, He Z, Djurišić AB, Yip CT, et al. (2007) Metallated conjugated polymers as a new avenue towards high-efficiency polymer solar cells. Nat Mater 6(7): 521-527.
- Mei J, Ogawa K, Kim YG, Heston NC, Arenas DJ, et al (2009) Low-band-gap platinum acetylide polymers as active materials for organic solar cells. ACS Appl Mater Interfaces 1(1): 150-161.
- Wong WY, Wang X, Zhang HL, Cheung KY, Fung MK, et al. (2008) Synthesis, characterization and photovoltaic properties of a low-bandgap platinum(II) polyyne functionalized with a 3,4-ethylenedioxythiophene-benzothiadiazole hybrid spacer. J Organomet Chem 693(24): 3603-3612.
- Wang Q, Wong WY (2011) New low-bandgap polymetallaynes of platinum functionalized with a triphenylamine-benzothiadiazole donor–acceptor unit for solar cell applications. Polym Chem 2(2): 432-440.
- Wong WY, Wang XZ, He Z, Chan KK, Djurišić AB, et al. (2007) Tuning the absorption, charge transport properties, and solar cell efficiency with the number of thienyl rings in platinum-containing poly(aryleneethynylene)s. J Am Chem Soc 129(46): 14372-14380.
- Wong WY (2007) Luminescent organometallic poly(aryleneethynylene)s: Functional properties towards implications in molecular optoelectronics. Dalton Trans 40: 4495-4510.
- Wang L, Yin L, Ji C, Zhang Y, Gao H, et al. (2014) High open-circuit voltage of the solution-processed organic solar cells based on benzothiadiazole–triphenylamine small molecules incorporating π-linkage. Org Electron 15(6): 1138-1148.
- McKay TJ, Staromlynska J, Wilson P, Davy J (1999) Nonlinear luminescence spectroscopy in a Pt:ethynyl compound. J Appl Phys 85(3): 1337-1341.
- Torre G, Bottari G, Sekita M, Hausmann A, Guldi DM, et al. (2013) A voyage into the synthesis and photophysics of homo- and heterobinuclear ensembles of phthalocyanines and porphyrins. Chem Soc Rev 42(20): 8049-8105.
- Liu R, Azenkeng A, Zhou D, Li Y, Glusac KD, et al. (2013) Tuning photophysical properties and improving nonlinear absorption of Pt(II) diimine complexes with extended π-conjugation in the acetylide ligands. J Phys Chem A 117(9): 1907-1917.
- Liu R, Li Y, Chang J, Waclawik ER, Sun W (2014) Pt(II) bipyridyl complexes bearing substituted fluorenyl motif on the bipyridyl and acetylide ligands: Synthesis, photophysics, and reverse saturable absorption. Inorg Chem 53(18): 9516-9530.
- Shelton AH, Price RS, Brokmann L, Dettlaff B, Schanze KS (2013) High efficiency platinum acetylide nonlinear absorption chromophores covalently linked to poly(methyl methacrylate). ACS Appl Mater Interfaces 5(16): 7867-7874.
- Boixel J, Guerchais V, Le Bozec H, Jacquemin D, Amar A, et al. (2014) Second-order NLO switches from molecules to polymer films based on photochromic cyclometalated platinum(II) complexes. J Am Chem Soc 136(14): 5367-5375.
- Wong WY (2009) Challenges in organometallic research-great opportunity for solar cells and OLEDs. J Organomet Chem 694(17): 2644-2647.
- Lam ESH, Tsang DPK, Lam WH, Tam AYY, Chan MY, et al. (2013) Luminescent platinum(II) complexes of 1,3-bis( N -alkylbenzimidazol-2′-yl)benzene-type ligands with potential applications in efficient organic light-emitting diodes. Chem A Eur J 19(20): 6385-6397.
- Forrest SR (2004) The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 428(6986): 911-918.
- Sheng CX, Singh S, Gambetta A, Drori T, Tong M, et al. (2013) Ultrafast intersystem-crossing in platinum containing π-conjugated polymers with tunable spin-orbit coupling. Sci Rep 3(1): 1-7.
© 2021 Rayya A Al Balushi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






