- Submissions

Full Text
Annals of Chemical Science Research
Chemical Design, Synthesis and Bio-efficacy Screening of New Growth Inhibitors of Spodoptera littoralis (Boisd.)
Mohamed A Gad*
Agricultural Research Center, Egypt
*Corresponding author: Mohamed A Gad, Agricultural Research Center, Egypt
Submission: October 9, 2019;Published: October 22, 2019

Volume1 Issue4October, 2019
Abstract
The present work aimed to find new growth inhibitors agents spodoptera littoralis (Boisd.), several inhibitors structurally relevant to the insect growth regulator, Fenoxycarb and the naturally transpiring juvenile hormone of insects were chemically designed, prepared and evaluated as anti-proliferative agents. Epihalohydrins derivatives have been synthesized and their agricultural bio-efficacy as insecticides against spodoptera littoralis (Boisd.). Insecticidal bio-efficacy data showed that that some compounds are very active against spodoptera littoralis (Boisd.)
Keywords: Insecticidal bio-efficacy; Spodoptera littoralis (Boisd.); Insect growth regulator; The toxicity ratio
Graphical Abstract

Introduction
Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) is considered one of the key pests that cause great damage to cotton plants as well as other plants in Egypt [1,2] larvae of this insect can feed on about ninety economically, monetarily and significant plant pertinence to forty families. The achieved control is not successful, when using synthetic insecticides [3] and some biorational agents for example Bacillus thuringiens is Berliner to resist the pest, because of the insect’s high ability to develop resistance toward most of traditional components [4]. Therefore, we need novel compounds that are efficient against this insect, safe to human and ecological well-disposed [5,6]. The substitutional control techniques that show presage as a potential tactic in S. littoralis the executive’s projects is the utilization of biorational control operators for example synthetic insect growth regulators and those dependent on normally materials [7]. Insect growth regulators are development controllers are ventured to be more secure for useful living beings than regular mixes, and they have been effectively utilized in IPM programs against many tree and little natural product [8,9]. There is a need for different insecticides having different modes of action Juvenile hormones analogues [10-13] sesquiterpenoid moieties arranged and discharged by the corpora allata, are significant insect hormones that standardize a bulky diversity of processes during postembryonic growth and adult reproduction in insects [14]. Juvenile hormone analogues display powerful creepy crawly adolescent hormone-mirror action, and consequently cause genuine aggravations in the advancement, multiplication, and conduct of a wide scope of bug [15-16]. This carbamate is utilized for pests’ control in farming, ranger service, and put away items, and is additionally utilized as a general wellbeing bug-spray Juvenile hormone analogue is recorded for control of caterpillars, and scales in Europe, and for flame ants in the U.S. Like most IGRs, fenoxycarb has surprisingly low human toxic quality and is significantly more bug particular than the ordinary bug sprays [17]. The main objective of this study was to determinate the toxicity of new growth inhibitors agents against the cotton leafworm, S. littoralis larvae under laboratory conditions.
Results and Discussion
The start reagent can be prepared by the reaction of 2-naphthol with epichorohydrin in presence of catalytic amount of sodium hydroxide afforded of 2-((2naphthyloxy) methyl)-oxiran1a (Figure 1-3).
Figure 1:The nucleophilic attack of different primary, secondary amines and hydroxyl group to the 2-[(naphthalen-2-yloxy)methyl] oxirane1a afford of 1-(naphthalen-2-yloxy)-3-(piperidin-1-yl)propan-2-ol1, 1-(morpholin-4-yl)-3(naphthalen-2-yloxy)propan-2-ol 2, 3, 3’-(piperazine-1,4-diyl)bis(1-(naphthalen-2yloxy) propan-2-ol)3,1,3-bis(naphthalen-2-yloxy)propan-2-ol4,1,3-is(naphthalen-2yloxy)propan-2-one 5,3,3’-(ethane- 1,2-diylbis(azanediyl))bis(1-(naphthalen-2yloxy) propan-2-ol) 6, 1 (butylamino)-3-(naphthalen-2-yloxy)propan-2- ol 7, 1- (hexylamino)-3-(naphthalen-2-yloxy) propan-2-ol 8, 3, 3’-(hydrazine-1,2-diyl) bis (1-(naphthalen-2-yloxy) propan-2-ol)9, 1-cyclopropyl-6-fluoro-7-(4-(2-hydroxy-3(naphthalen-2-yloxy)propyl)piperazin-1-yl)-4-oxo-1, 4-dihydroquinoline-3-carboxylic acid 10 and 3,3’,3’’-nitrilotris(1-(naphthalen-2-yloxy)propan-2-ol) 11, respectively
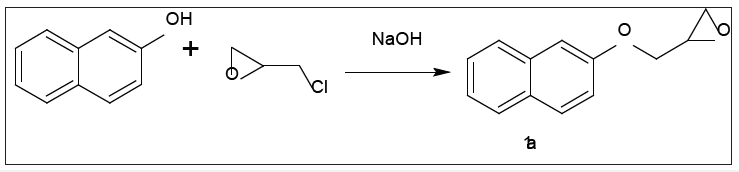
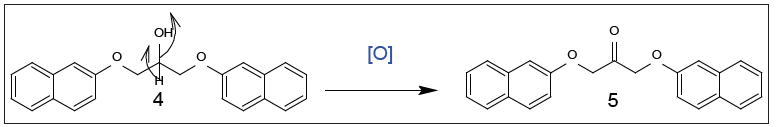
Figure 2:Further compound 5 undergoes oxidation reaction through targeted with benzoyl chloride in the presence of sodium ethoxide in absolute ethanol yield compounds 5 which were emphasized by spectral and elemental analyses. IR spectrum demonstrated absorption bands at 1701cm-1 for (C=O) in which replaced by bands of (OH) at 3290cm-1. 1HNMR (DMSO-d6) of component 5 explicated singlet signals at 4.3 for (CH2) and 4.6 for (CH2) groups. The group of the (OH) of component 4 was vanished when reacted to afford component 5 while 13C- NMR (DMSO-d6), showed (C=O) at δ 174.06 and δ 36.82, 46.35 for 2(CH2), respectively (Figure 3).
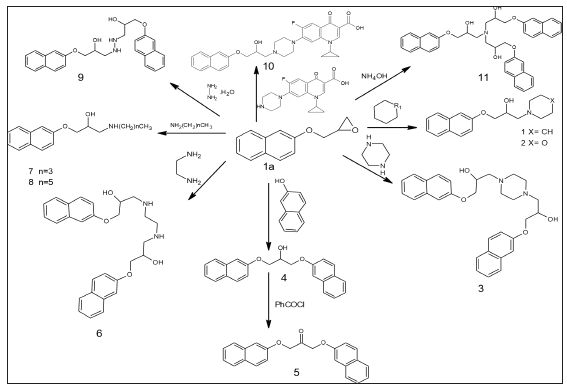
Figure 3:
Experimental
A Fisher-Johns apparatus was exercised to register the MP. of every synthesized compound. Infra-red and elemental analyses (C, H, N, and S) were accomplished through a Pye Unicam SP3-100 spectrophotometer utilizing the KBr disk manner and a Vario EL C, H, N, S analyzer, separately. A Bruker 400MHz spectrometer was utilized to measure DEPT 135 spectra and the 1H and 13C NMR spectra within the TMS as an interior standard. Chemical shifts were estimated in ppm. Reaction headway and perfection of the prepared sections were checked by thin layer chromatography. The insecticidal reference, fenoxycarb (insect growth regulators), was achieved from Sigma Aldrich (France), spodopetra littoralis (Boisd) were combined from cotton area of the exploratory farm of Agricultural research center. The insecticidal effective of the reported fenoxycarb juvenile hormones as active ingredient and the prepared components was screened against larvea of the spodopetra littoralis (Boisd).
Synthetic Procedure for 2-[(naphthalen-2-yloxy) methyl] oxirane(1a). According to the reported procedure, by reaction of 2-naphthol(0.04mol) with epichlorohydrin (0.12mol) and sodium hydroxide 25% in water was stirred in ice bath for 4 h, compound 1a was prepared, The formed precipitate was filtered off and recrystallized from methanol in which give white powder of 1a. Yield: 81%; MP.92-94 °C. IR (ν) (KBr) cm−1, 3049 (C−H aromatic), 2913, 2880 (C−H aliphatic); 1H NMR (DMSO-d6): δ6.7 (m, 7H, Ar−H), 4.47, 3.91, 3.41,2.77, 2.76 for (s. 1H, CH), respectively. Elemental analysis calculated for C13H12O2 (%) Calcd. /found C, 77.98/77.97; H, 6.04/6.02.
General Procedure of Synthetic 3-Cyano-4, 6-distyrylpyridine- 2(1H)-thione (1-4, 611). A mixture of compound 1a (1mmol), nucleophile (3mmol) and drops of tri ethylamine in ethanol was stirred and refluxed at ambient temperature for 5h to give a precipitate which filtered off and recrystallized from 1,4-Dioxan. 1-(naphthalen-2-yloxy)-3-(piperidin-1-yl) propan-2-ol (1) Pale red crystals. Yield: 91%; MP: 119− 122 °C. IR (ν) (KBr) cm−1: 3362 (OH), 2934, 2915, 2824 (C−H aliphatic). 1H NMR (DMSO-d6): δ7.19−7.89 (m, 7H Ar−H), 4.72 (s, 1H, OH),4.11 (s, 1H, CH), 4.00 (m, 4H, 2CH2), 1.5 (m. 10H, 5CH2).13C NMR (DMSOd6): δ 157.20, 134.81, 129.69, 127.21, 126.77, 123.92, 119.2, 107.25, 71.78, 70, 28, 67.00, 55.32, 26.19, 24.64. Elemental analysis calculated for C18H23NO2 (%) Calcd./found C, 75.76/75.77; H, 8.12/8.11; N, 4.91/489.
1-(morpholin-4-yl)-3-(naphthalen-2-yloxy) propan-2-ol (2)
Yellow crystals in 59% yield, M.P112-115 °C. IR(ν) (KBr) cm−1: 3316 (OH), 3273(NH), 3080 (C−H aromatic), 2989, 2879 (C−H aliphatic). 1H NMR (DMSO-d6): δ7.18−7.89 (m, 7H Ar−H), 4.72 (s, 1H, OH), 4.1 (s, 1H, CH), 4.00(s, 4H, 2CH2), 1.5(m. 8H, 4CH2). 13C NMR (DMSO-d6): δ 157.20, 134.81, 129.69, 127.94, 127.21, 126.03, 125.77, 107.25, 71.78, 67.00, 62.36, 26.19, 24.64. Elemental analysis calculated for C17H21NO3 (%) Calcd. /found C, 71.06/71.05; H, 7.37/7.36; N, 4.87/4.86.
3,3’-(piperazine-1,4-diyl) bis(1-(naphthalen-2-yloxy) propan- 2-ol) (3)
Yellowish white powder 87% yield, MP.188-190 °C. IR (ν) (KBr) cm−1: 3377 (OH), 2925, 2815 (C−H aliphatic), 1H NMR (DMSO-d6): 7.18−7.89 (m, 14H Ar−H). 4.84 (s, 2H, OH), 4.13(s, 2H, 2CH), 4.01 (s. 8H, 2CH2), 2.3(s. 8H, 4CH2).13C NMR (DMSO-d6): δ 157.16, 134.78, 129.71, 127.94, 126.80, 123.96, 107.27, 71.61, 67.01, 61.61, 54.13. Elemental analysis calculated for C30H34N2O4 (%) Calcd. /found C, 74.05/74.03; H, 7.04/7.02; N, 5.76/5.77.
1,3-bis(naphthalen-2-yloxy) propan-2-ol (4)
White powder 79% yield, MP. 111-113 °C. IR (ν) (KBr) cm−1: 3293 (OH), 3056, 3026 (C-H aromatic), 2928, 2877 (C−H aliphatic), 1H NMR (DMSO-d6): δ 7.21-7.85 (m, 14H, Ar−H), 5.56 (s, 1H, OH), 4.42 (s, 1H, CH), 4.25 (s, 4H, 2CH2).13C NMR (DMSO-d6): δ 139.20, 136.21, 133.69, 132.35, 128.85, 128.77, 125.32, 37.3, 45.25. Elemental analysis calculated for C23H20O3 (%) Calcd. /found C, 80.21/80.19; H, 5.8/5.7.
Synthetic procedure for 1,3-bis(naphthalen-2-yloxy) propan- 2-one (5)
Solution of compound 4 (1g, 0.0029mmole) from ethanol absolute (10ml) added to sodium ethoxide solution (0.06 gram, 0.0025mmole) and benzoyl chloride (0.49gram, 0.0025mmole) then refluxed for 5 hours. The product was formed are filtered off and recrystallized from methanol to afford compounds 5. Yellowish white in 51% yield, MP. 162-164 °C. IR (ν) (KBr) cm−1: 2897 (C-H aliphatic), 1701 (C=O). 1H NMR (DMSOd6): δ7.21-7.76 (m, 14H Ar- H),4.5 (s, 2H, CH2), 4.4 (s, 2H, CH2). 13C NMR (DMSO-d6): δ 174.06, 166.42, 136.30, 133.67, 132.35, 129.50, 129.04, 128.95, 128.73, 36.82, 46.35. Elemental analysis calculated for C23H18O3 (%) Calcd. /found C, 80.68/8067; H, 5.30/531.
3 ,3’-(ethane-1,2-diylbis(azanediyl))bis(1-(naphthalen-2- yloxy)propan-2-ol ) (6)
Yellowish powder in 59 % yield, MP. 219-222 °C. IR (ν) (KBr) cm−1: 3341 (OH), 3325 (NH), 3033 (C−H aromatic), 2922 (C−H aliphatic). 1H NMR (DMSO-d6): δ 7.12−7.81 (m, 2H, 2NH and 14H, Ar−H), 5.05 (s, 2H, 2OH), 4.12 (m, 2H, 2CH and 8H, 4CH2), 3 2.8 (s, 4H, 2CH2). 13C NMR (DMSO-d6): δ157.20, 133.90, 128.06, 126.90, 124.15, 123.14, 119.29, 71.39, 57.22 31.73, 22.98. Elemental analysis calculated for C26H28N2O4 (%): Calcd./found C, 73.02/73.01; H, 7.0/07.02; N, 6.08/6.06.
1-(butylamino)-3-(naphthalen-2-yloxy) propan-2-ol (7)
White powder in 88% yield, MP. 233-236 °C. IR (ν) (KBr) cm−1: 3496 (OH), 3024 (C−H aromatic), 2920, 2819 (C−H aliphatic). 1H NMR (DMSO): δ 7.12−7.71 (m, 1H, NH and 7H, Ar−H), 4.95 (s, 1H, OH), 4.12 (s, 4H, CH), 2.9 (s, 1H, CH), 4.82 (s, 3H, CH), 4.82 (s, 1H, CH), 4.82 (s, 1H, CH), 4.82 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 157.20, 134.70, 129.94, 1.28.96, 127.89, 127.05, 126.69, 123.89, 119.15, 107.2, 71.17, 67.86, 58.64, 55.78, 29.50, 20.54, 14.25. DEPT 135 (DMSO-d6): δ 129.56 (CH), 127.90 (CH), 127.06 (CH), 126.7 (CH), 123.90 (CH), 119.18 (CH), 107.14 (CH), 106.9 (CH), 71.17 (CH), 70.97 (OCH), 14.33(OCH2). Elemental analysis calculated for C17H21NO2 (%) Calcd. /found C, 74.69/74.77; H, 8.48/8.47; N, 5.12/5.13.
1-(hexylamino)-3-(naphthalen-2-yloxy) propan-2-ol (8)
Pale white powder 73% yield, MP. 123−126 °C. IR (ν) (KBr) cm−1: 3313 (OH), 3024 (C−H aromatic), 3055, 3029 (C-H aromatic), 2920 (C−H aliphatic). 1H NMR (DMSO-d6): δ 7.12−7.81 (m, 7H, 7H, Ar−H), 4.25 (s, 1H, OH), 4.12 (s, 2H, CH), 2.8 (s, 1H, CH), 2.7 (s, 1H, CH), 2.6 (s, 2H, CH), 1.44 (s, 2H, CH), 1.42 (s, 6H, CH), 1.2 (s, 3H, CH3). 13C NMR(DMSO-d6,): δ157.14, 134.80, 129.66, 1.28.96, 127.92, 127.11, 126.75, 123.92, 119.21, 107.26, 71.39, 68.55, 52.97, 49.96, 31.73, 29.98, 26.92, 22.55, 14.33. Elemental analysis calculated for C19H25NO2 (%) Calcd. /found C, 75.71/75.70; H, 9.03/9.05; N, 4.65/4.63.
3,3’-(hydrazine-1,2-diyl) bis(1-(naphthalen-2-yloxy) propan- 2-ol) (9)
Yellowish powder in 59 % yield, MP. 219-222 °C. IR (ν) (KBr) cm−1: 3340, 3323 (OH, NH), 3039 (C−H aromatic), 2924 (C−H aliphatic). 1H NMR (DMSO-d6): δ 9.71 (s, 2H, 2NH), 7.18-7.81 (m, 28H, Ar−H), 5.11 (s, 2H, 2OH), 4.51 (m, 2H, 2CH), 4.15(m, 8H, 4CH2). 13C NMR (DMSO-d6): δ157.11, 134.78, 129.69, 127.93, 126.78, 123.96, 107.28, 71.36, 68.80, 53.25. Elemental analysis calculated for C26H28N2O4 (%) Calcd. /found C, 72.20/72.18; H, 6.53/6.52; N, 6.48/6.49.
1-cyclopropyl-6-fluoro-7-(4-(2-hydroxy-3-(naphthalen-2- yloxy)propyl)piperazin-1yl)-4-oxo-1,4- dihydroquinoline-3- carboxylic acid(10)
Pal white powder in 70% yield, MP. 210-212 °C. IR (ν) (KBr)cm- 1: 3344 (OH), 3018(C−H aromatic), 2957, 2863C−H aliphatic), 1726 (C=O). 1H NMR (DMSO-d6): δ 14.60 (OH), 8.46 (NH), 7.12−7.71 (m, 10H, Ar−H), 4.95 (s, 1H, OH), 4.12-3.81 (m, 5H, CH), 2.6 (d, 8H, CH2), 1.12 (m, 5H, CH). 13C NMR (DMSO-d6,): δ 157.20, 134.70, 129.94, 1.28.96, 127.89, 127.05, 126.69, 123.89, 119.15, 107.2, 71.17, 67.86, 58.64, 55.78, 29.50, 20.54, 14.25. Elemental analysis calculated for C30H30FN3O5 (%) Calcd. /found C, 67.78/67.76; H, 5.69/5.68; N, 7.90/7.91.
3,3’,3’’-nitrilotris(1-(naphthalen-2-yloxy) propan-2-ol) (11)
Pale White powder in 92% yield, MP. 138-140 °C. IR (ν) (KBr) cm-1: 3221 (OH), 2919, 2813 (C−H aliphatic). 1H NMR (DMSO-d6): δ 7.12-7.81 (m, 21H, Ar-H), 5.04 (s, 3H, OH), 4.12 (m, 6H, CH2 and 3H, CH), 2.9 (m, 6H, CH2).13C NMR (DMSO-d6): δ 157.11, 134.78, 129.70, 127.94, 123.97, 119.23, 107.28, 73.02, 69.80, and 53.2. Elemental analysis calculated for C39H39NO6 (%) Calcd. /found; C, 75.83/75.82; H, 6.36/6.35; N, 2.27/2.28.
Laboratory Bioassay
The insecticidal bio-efficacy of all prepared Epihalohydrins analogues was assessed by the leaf dipping bioassay procedure. Research facility of tested results are accounted here for the used compounds to discover the appropriate concentration that are required to kill half 50% (LC50) of the pests. In this search, five concentrations of each synthesized naphthalene derivatives and 0.1% Triton X-100 as surfactant were utilized. A number total of 10 larvae of spodoptera littoralis (Boisd.), almost of the 2nd instar larvae and 4th instar larvae size, Disks (9cm. diameter) of castor bean leaves were dipped in the tested concentrations for 10 seconds then left to dry and offered to larvae. Larvae were placed into glass jars (5 pounds), every treatment was recreated multiple times (10 larvae per each). Control disks were dunked in distilled water only. In which allowed the larvae to feed on castor bean leaves for 48hr [18]. then transferred to the untreated ones. Mortality percentages were recorded after 72hr. for all insecticides. Mortality was redressed by Abbott’s formula [19]. The measurements mortality relapse lines were measurably dissected by probit analysis [20]. Harmfulness Index was determined by sun equations [21].
Insecticidal Bio-efficacy Examination
Table 1:Insecticidal activity of Compounds 1−11 and Fenoxycarb against the 2nd larvae of spodoptera littoralis (Boisd.), after 72h of Treatment.
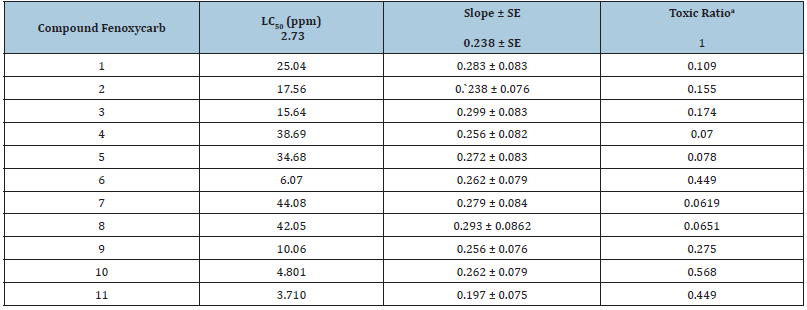
aThe ratio of the LC50 values of fenoxycarb for baseline toxicity and the compounds are defined as a toxic ratio.
Table 2:Insecticidal activity of compounds 1-11and Fenoxycarb against the 4th larvae of spodoptera littoralis (Boisd.), after 72h of Treatment.

The compounds which prepared have been tested for their insecticidal bio-efficacy, demonstrated as follows: compounds from 1-11 were tested against the 2nd instar larvae for their activity as insecticidal, and the outcomes are introduced in Table 1. After 72h of treatment, activity results demonstrated that these compounds show high to low insecticidal activity against the 2nd instar larvae and the LC50 regards stretched out from 3.71 to 44.08ppm, however the LC50 estimation of fenoxycarb was 2.37ppm. This shows that nearly insecticidal bio-efficacy of fenoxycarb as insect growth regulators after 72h of test. In which compounds 6, 10 and 11 have generally excellent insecticidal bio-efficacy against 2nd because that their LC50 esteems were 6.07, 4.80 and 3.710, respectively, in which the LC50 estimation value of fenoxycarb was 2.37ppm.
The results of insecticidal bio-efficacy after 72h for compounds 1-11 were tested against the 4th instar larvae shown in Table 2. Varied from strong to weak with LC50 values assorted from 50.60 to 103.01ppm, while the LC50 value of fenoxycarb was 42.231ppm. In which demonstrates that various them have tested compound near that of fenoxycarb as juvenile hormone after 72h of test. For instance, compounds 6, 10 and 11 have generally excellent insecticidal exercises activities against 4th instar larvae because their LC50 values were 54.631, 57.622 and 50.606, respectively, and the LC50 value of fenoxycarb was 43.231 ppm.
Structure Framework Relationship
It shows up from the general search structure of the prepared naphthalene derivatives compounds of 11and 10are more active against 2nd instar larvae and 4th instar larvae than the another’s of tested compounds. It additionally demonstrated that exacerbates that contain an exceptionally ether group in the structure claim a high insecticidal bio-efficacy. Thus, compounds 6, 9, 10, and 11 more active than compounds 1, 2, 3, 4, 7 and 8 in-insecticidal bioefficacy.
References
- Marwa FK, Ali AM (2017) Impact of some essential plant oils and insect growth regulators on immature stages of spodoptera littoralis (Boisd.). Lepidoptera: Noctuidae 8(11): 561-570
- Amira SMI (2019) Sterilizing activity of the insect growth regulator, lufenuron on drosohpila melanogaster (Meigen). J Plant Prot and Path 10(5): 297-302.
- GelbicI, Adel MM, Hussein HM (2011) Effects of nonsteroidal ecdysone agonist RH-5992 and chitin biosynthesis inhibitor lufenuron on Spodoptera littoralis (Boisduval, 1833). Central European Journal of Biology 6: 861-869.
- Nasr HM, Badawy MEI, Rabea EI (2010) Toxicity and biochemical study of two insect growth regulators, buprofezin and pyriproxyfen, on cotton leafworm Spodoptera littoralis. Pestic Biochem Physiol 98(2): 198-205.
- Wheeler DE, Nijhout HF (2003) A perspective for understanding the modes of juvenile hormone action as a lipid signaling system. Bio Essays 25(10): 994-1001.
- Niu JJ, Meng Q, Chai HY, Chu KH, et al. (2019) Effects of two juvenile hormone analogue insecticides, fenoxycarb and methoprene on neocaridina davidi. Environmental Pollution 253: 89-99.
- El Sheikh EA, Kamita SG, Vu K, Hammock BD (2011) Improved insecticidal efficacy of a recombinant baculovirus expressing mutated JH esterase from Manduca sexta. Biol Control 58(3): 354-361.
- Bortolotti L, Porrini C, Sbrenna AM, Sbrenna G (2000) Ovicidal action of fenoxycarb on a predator, Chrysoperla carnea. Appl Entomol Zool 35(2): 265-270.
- Khalil SMS, Anspaugh DD, Roe RM (2006) Role of juvenile hormone esterase and epoxide hydrolase in reproduction of the cotton bollworm helicoverpa zea. J Insect Physiol 52(7): 669-678.
- Wilson TG (2004) The molecular site of action of juvenile hormone and juvenile hormone insecticides during metamorphosis: how these compounds kill insects. J Insect Physiol 50(2-3): 111-121.
- Retnakaran A, Granett J, Ennis T (1985) Ennis Insect growth regulators. In: Kerkut G, Gilbert LI (Eds.), Comprehensive Insect Physiology, Biochemistry and Pharmacology 12, Pergamon Press, New York, USA, pp. 529-601.
- Dedos SG, Szurduki F, Skarlatos Székácsc A, Shiotsuki T, Hammock BD, et al. (2002) Fenoxycarb levels and their effects on general and juvenile hormone esterase activity in the hemolymph of the silkworm, bombyx mori. Pesticide Biochemistry and Physiology 73(3): 174-187.
- Staal GB (1975) Insect growth regulators with juvenile hormone activity. Annu Rev Entomol 20: 417-460.
- Wael LD, Greef MD, Laere OV (1995) Toxicity of pyriproxifen and fenoxycarb to bumble bee brood using a new method for testing insect growth regulators. J Apicultural Research. 34(1): 3-8.
- Riddiford LM (2008) Juvenile hormone action: a 2007 perspective. J Insect Physiol 54(6): 895-901.
- Zera JA, Tanaka S (1996) The role of juvenile hormone and juvenile hormone esterase in wing morph determination in Modicogryllus confirmatus. J Insect Physiology. 42(9): 909-915.
- Abdel-Aal YAI, Hammock BD (1986) Transition state analogs as ligands for affinity purification of juvenile hormone esterase. Science 233(4768): 1073-1076.
- Kamita SG, Hammock BD (2010) Juvenile hormone esterase: biochemistry and structure. J Pestic Sci 35(3):265-274.
- Abbott WS (1987) A method of computing the effectiveness of an insecticide. J Econ Entomol. 18:265-267.
- Finney DJ (1952) Probit analysis a statistical treatment of the sigmoid response curve, cambridge university press. Cambridge, USA 116 (3011): 286-287
- Sun YP (1950) Toxicity index-An Improved method of comparing the relative toxicity of insecticides. J Econ Entomol 43 (1): 45-53.
© 2019 Mohamed A Gad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






