- Submissions

Full Text
Annals of Chemical Science Research
Toxicity Alert: Hydrogen Sulfide
Pipat Chooto*
Department of Chemistry, Faculty of Science, Prince of Songkla University, Thailand
*Corresponding author:Pipat Chooto, Department of Chemistry, Faculty of Science, Prince of Songkla University, Thailand
Submission: September 02, 2019;Published: September 06, 2019

Volume1 Issue4September, 2019
Abstract
Recent cases of casualty of workers in rubber latex factories due to hydrogen sulfide call for urgent attention to better understanding, prevention of damages in working and living in risky environments, and promotion of public and labor awareness. Specific responsible organization here is the subcontractor of latex factories. H2S characteristics, sources and symptoms from exposure are emphasized. Proper practice should be followed strictly along with better cooperation among experts for the main objective in saving lives and greater safety in living and working.
Keyword: Industrial pollutants; Casualty from toxic gases; Toxicity awareness; Cooperations and practice for gas safety; Environmental pollutants
Introduction
The way that there are greater and greater number of industrial factories especially petroleum and rubber together with greater amount of wastewater, garbage and pollution causes casualty loss as well as health problems repeatedly. Lately in southern Thailand, there is the case that workers did their jobs in wastewater treatment areas, causing loss of consciousness, serious harm and death due to hydrogen sulfide [1]. Therefore, greater recognition and awareness of this gas are essential which can lead to the prevention as well as careful plan for the operation to make it safer for life in the future.
H2S properties
Hydrogen sulfide is the compound of hydrogen and sulfur with the chemical formula of H2S, similar to water (H2O) so it can dissolve in water very well. It is colorless with the odor of rotten eggs and greater density than air. After contact, the olfactory reception is paralyzed and cannot sense the gas any more, intensifying its toxicity [2]. The gas can be found everywhere in this whole wide world. It is toxic, corrosive, flammable and acidic when dissolving in water. For reading convenience, H2S is used instead of hydrogen sulfide from this point on.
H2S sources
In nature, hydrogen sulfide is found in great percentage in natural gas. It is obtained from volcano eruption and deserted wells. By chemical reactions, it can be formed by the reaction of hydrogen and sulfur at high pressure and temperature, the reactions of sulfide compounds with acids and the decomposition of certain sulfur compounds such as thioacetamide (CH3CSNH2). For the waste from communities or factories, it arises from the digestion by anaerobic bacteria which are normally bacteria consuming sulfate to get energy.
Existence in environment
For natural environment, due to the fact that the process of becoming fuel source involves sulfur, H2S can be found in various areas. Not large amount is found in petroleum and petroleum products. In contrast, it can be found at high concentration up to 90% in natural gas. It can also move from underground to the surface via volcano and hot or cold spring. And because of bacteria, deserted wells can also be filled with H2S. Finally, smaller amount of hydrogen sulfide can be formed in specific organs of human and animal bodies, mainly mouth and intestine, by the decomposition of proteins by bacteria.
In the case of industrial environment, H2S can be found in petroleum distillation from the reactions to remove sulfur. A number of industries that give away H2S to the environment include coal burning, paper mills, tanning, sewerage and mining among others. The way that sulfur is left to react with organic matters at high temperature can also lead to the formation of H2S by bacterial decomposition. Finally, oil drilling can cause the releasing of this gas to the environment at the surface. People in the community generally get contacted with hydrogen sulfide from wastewater and wastewater treatment systems, nearby factories, the areas for landfills and manure storage and processing.
Specific industry for H2S
Hydrogen sulfide can specifically be found in farms, fishery boats, petroleum industry, and latex factories. The first place is compost containers keeping manure of pigs or cows in the farm. It also exists in wastewater treatment tanks or draining pipes as well as in the bilges of fishing boats which are used to keep fish and the fish might deteriorate and release the gas. The rice mills or corn warehouse with the conveyer to transport rice or corn can have hydrogen sulfide because some rice or corn might spill and pile up. The processes in obtaining and distilling petroleum and natural gas normally have to deal with fossils and get hydrogen sulfide as a by-product [2]. However, the recent case of the factory in southern Thailand occurred from H2S in wastewater systems of a latex factory.
H2S in latex factories
It is beyond expectations that latex factories can be harmful due to the process of making concentrated latex and in turn wastewater treatment. From Latex, the factories make use of centrifugation to obtain 60% concentrated latex. However, the diluted parts which are called skim latex are considered profitable because they contain certain amount of rubber. Sulfuric acid is applied for its coagulations to obtain the so-called skim rubber. Consequently, wastewater from this process is acidic and contains sulfate from which hydrogen sulfide is formed when sulfate is digested by anaerobic bacteria so that they can gain energy. The concentration of sulfide can be very high, up to 12,000 ppmv therefore it can be risky for the workers particularly those working on wastewater treatment [3,4].
Special case of concentrated latex factories
Latex factories are prominent in southern Thailand and they are presently found increasingly all over Thailand due to widespread rubber plantations. Recently in southern Thailand, there was the case that 3 workers dead, 4 losing consciousness and 1 seriously hurt [1]. The case was confirmed to be due to hydrogen sulfide, hence the inspirations for toxicity alert articles. It is also shocking to find that there had been a number of casualty cases before. One of the ways to prevent this is better public recognition, understanding and awareness of H2S. However, there are specific items to be considered in this special case in southern Thailand. First, it was not the company, but instead the subcontractor, who took care of the job of fixing wastewater treatment systems. Secondly, the people who worked there have no specific duty to do that job. They just helped the responsible workers, their friends who were from the same hometown, who behind-the-scene informed that before the operation as assigned, they secretly heard the warning not to go for the work but that is another separate story. Thirdly, it was found that the oxygen tubing of protection equipment was leaking. All in all, the knowledge about handling the areas with the presence of H2S is urgently, seriously and extremely required.
Toxicity of H2S
H2S had special mechanism and symptoms especially to our body. To lead to toxicity, it works by reacting with specific enzyme namely cytochrome oxidase and blocking its functions. The cells therefore cannot obtain the energy from the process of energy transformation or respiration. Furthermore, the oxygen contact and intake are certainly less due to less surface areas from the replacement by H2S. Due to the fact that H2S affects the metabolism of the cells, general symptoms would start from the irritation of all types of bodily membranes.
Levels of toxicity and symptoms
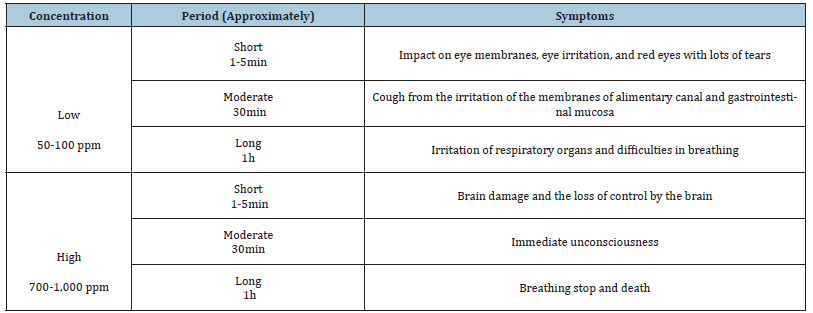
The symptoms of those exposed to H2S depend on both the concentration and time of contact. Normally the minimum allowance is 1 ppm for 8 hours (an eight-hour time weighted average, TWA) and 5 ppm for 15 minutes (15-minute short-term exposure limit, STEL). It has to be emphasized here that the toxicity would be greater if the exposure is in the limited space without proper ventilations which would result in acute symptoms or sudden death therefore the toxicity is also reported in terms of weight/volume. The main body response to hydrogen sulfide is concisely presented in Table 1.
Table 1:E studies about alternative therapy.
Things to be done in factories
To help the situation of H2S in factories, there are five top priority measures to be implemented. First is the ventilation; the factories need to have have good facilitation for air flow. The workers also need to wear the clothes that are not too tight and well-ventilated. Secondly, the oxygen masks are required together with the equipment to prevent the chemicals and goggles. Thirdly, the workers need to be well educated and trained with the certificates to let others know that they are well qualified. Fourth, there must be the safety equipment at the working sites. Finally, there must be the sensors for hydrogen sulfide and the equipment to signal the warning in case the concentration exceeds the the limit as well as security tools are necessary to be installed. If the case is worse than the aforementioned, then the air needs to be sampled and measured continuously by flow-based sensors to make sure about the amount of toxic gases.
Determination of H2S
H2S can be determined by a number of methods recently including gas chromatography (GC) [9], optical probes [10], emission spectrometry [11], cyclic voltammetry [12] and fluorescent probes [13,14]. The most used on site is electrochemical methods [5]. As a matter of fact, this is one of the research topics proposed by the student at the author’s electrochemical research lab due to the fact that wastewater from rubber sheet cleaning was found to have certain harmful effects plants of citrus family, especially lime. so it deserves separate review articles for those who specifically focus on recent H2S analytical methods and their characteristics.<./
Lessons from the case of casualty
There are a number of lessons to be learned from the case of the damage from H2S to workers. Above all, the phrase “Safety First” is to be strictly held on to, which means carefulness in operation as well as common sense and awareness. In Thailand in particular, the way that people take it easy is normally a basic way of living, but it has to be emphasized that this is not the case because it concerns life and death. Even in other cases such as welding, the way that the welders do not use the masks can be easily seen. And due to the fact that Thai people normally follow local wisdom, at least they can think of the way that traditional well diggers use the candle to check the availability of oxygen. If the light goes out, it means the air is not safe for them anymore. The second thing that is the weak point especially in Thailand is maintenance. Everything needs to be well equipped according to the rules and regulations. Money has to be invested and things must be well planned on the security.
Thirdly, educating and training workers are required to allow them to be able to take care of themselves and others. Knowledge transfer to provide awareness to both workers and the public is necessary, which also reflects the importance of science subjects in school, particularly sulfur cycle in the environment. Next, the news warns us to take really good care of the environment. More often than not, widespread rubber latex companies especially in southern Thailand release the smell from time to time, particularly in the morning. It is surely irritating at least to a certain extent in particular to those who exercise and wake up early in the morning to go to work. No one knows about the accumulating effects that the smell might have and not to mention the rotten smell of wastewater. This is the perfect example to ask whether it is about time to take care of air, water or soil. The fifth to be well implemented is the integrated cooperation from all experts involved including engineers, chemists, environment personnel, medical doctors, industrialists, experts for rubber and polymer, public relations personnel, labor and administrators. Finally, everyone needs to be aware of significance of life and living. Our lives are of great value to families, society and the nation hence they should be well taken care of and highly valued. Real actions need to be taken and make the situations better right in time, before more harm and greater risks to become the next casualty cases.
Acknowledgement
The author wish to thank Dr. Walailak Puetpaiboon, the head of Department of Chemistry, Faculty of Science, Prince of Songkla University (PSU), Thailand; Dr. Morakot Kaewpet, a lecturer in Department of Chemistry, PSU and Mr. Isarapab Chumraksa, Public Relations (PR) officer from Faculty of Science, PSU, for the cooperation in airing the case to the public via radio interview by PSU Radio FM 88 Hatyai. Deep appreciation is also expressed to Asst. Prof. Dr. Ekwipoo Kalkornsurapranee, Deputy Director of Natural Rubber Innovation Research Institute, PSU, for information about rubber latex processes which proves that PSU is always there for all sorts of academic consultation. Thanks to PR division of PSU, the article in Thai was also sent to local newspapers to increase awareness impact. Last but not least, the gratitude is to be paid to Hatyai Cable 93 for the news details.
References
- https://www.youtube.com/watch?v=tn4Zv9NMb9s
- Jiang J, Chan A, Ali S, Saha A, Haushalter KJ, et al. (2016) Hydrogen sulfide-mechanisms of toxicity and development of an antidote. Sci Rep.
- Chaiprapat S, Wongchana S, Loykulnant S, Kongkaew C, Charnnok B (2015) Evaluating sulfuric acid reduction, substitution, and recovery to improve environmental performance and biogas productivity in rubber latex industry. Process Safety and Environmental Protection 94: 420-429.
- Promnuan K, O-Thong S (2017) Biological hydrogen sulfide and sulfate removal from rubber smoked sheet wastewater for enhanced biogas production. Energy Procedia 138: 569-574.
- https://ohsonline.com/articles/2011/09/01/monitoring-h2s-to meet-new-exposure-standards.aspx
- https://www.sanook.com/news/2553786/
- https://med.mahidol.ac.th/poisoncenter/th/poiscov/gas/Hydrosul
- http://www.euro.who.int/__data/assets/pdf_file/0019/123076/AQG2ndEd_6_6Hydrogensulfide.PDF
- Thompson R, Perry JD, Stanforth SP, Dean JR (2018) Rapid detection of hydrogen sulfide produced by pathogenic bacteria in focused growth media using SHS-MCC-GC-IMS. Microchemical Journal 140: 232-240.
- Luo Y, Zhu C, Du D, Lin Y (2019) A review of optical probes based on nanomaterials for the detection of hydrogen sulfide in biosystems. Analytica Chimica Acta 1061: 1-12.
- Wu Z, Jiang J, Li N (2015) Cold excitation and determination of hydrogen sulfide by dielectric barrier discharge molecular emission spectrometry. Talanta 144: 734-739.
- Nechaeva D, Shishov A, Ermakov S, Bulatov A (2018) A paper-based analytical device for the determination of hydrogen sulfide in fuel oils based on headspace liquid-phase microextraction and cyclic voltammetry. Talanta 183: 290-296.
- Wang H, Wu X, Yang S, Tian H, Liu Y, et al. (2019) A dual-site fluorescent probe for separate detection of hydrogen sulfide and bisulfite. Dyes and Pigments 160: 757-764.
- Wang H, Li Y, Yang S, Tian H, Liu Y, et al. (2019) A dual-function fluorescent probe for discriminative detection of hydrogen sulfide and hydrazine. Journal of Photochemistry and Photobiology A: Chemistry 377: 36-42.
© 2019 Abu-Dief AM. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






