- Submissions

Full Text
Annals of Chemical Science Research
Azomethine Metal Chelates as an Efficient Catalyst for Oxidation of Organic Compounds
Abu-Dief AM*
Chemistry Department, Faculty of Science, Sohag University, Egypt
*Corresponding author:Abu-Dief AM, Faculty of Science, Egypt
Submission: May 11, 2019;Published: June 11, 2019

ISSN : 2688-8394 Volume1 Issue3
Abstract
Azomethine ligands and their metal chelates are flexible compounds synthesized from the condensation of an amino compound with carbonyl compounds and extensively used for industrial purposes and also show a broad range of biological efficiencies including antibacterial, antifungal, antiviral, antimalarial, antiproliferative, anti-inflammatory, anticancer, anti-HIV, anthelminthic and antipyretic properties. Azomethine metal chelates show excellent catalytic activity in diversity reactions. Over the past few years, there have been many reports on their applications in homogeneous and heterogeneous catalysis. The high thermal and moisture stabilities of many azomethine metal chelates were useful attributes for their application as catalysts in reactions involving at high temperatures. Recent researches in oxidation catalysis have focused on how to employ the metal‐catalyzed oxidation of organic substrates. This review concerns with the current developments for the oxidations of organic compounds.
Keywords:Catalyst; Oxidation; Azomethine ligands; Metal chelates; H2O2
Introduction
Azomethine compounds played an important role as ligands due to their excellent coordinative capability and have a wide variety of industrial applications in many fields including analytical, biological, coordination inorganic chemistry and organic synthesis, such as pigments, dyes and catalyst intermediates, semiconductors and as chemo-sensors [1- 15]. The chemistry of azomethine ligands is an important area of research with increasing interest due to the simple synthesis, versatility and diverse ranges of applications of their metal chelates, e.g. in biology and as catalysts in various reactions [16-20]. Thus, a review highlighting the oxygen affinity of azomethine metal chelates would be needed.
Oxidation of organic compounds
Life would not exist without oxidation, which is very important from scientific and practical points of view. In organic chemistry, oxidation reactions are some of the most important ones. Thus, selective catalytic oxidations represent a severe challenge for modern organic chemistry, and their importance has been highlighted in terms of Nobel Prizes. The reaction between ONS donor ligands H2L (derived from 2- hydroxy benzaldehyde or its derivatives or 2‐hydroxy‐1‐naphthaldehyde and o‐amino-thiophenol) and [RuHCl(CO) (PPh3)] afforded new Ru(II) metal chelates of [Ru(L)(CO) (PPh3)] (L=dianionic ONS donor Azomethine ligand). Theses metal chelates were found to be effective for the oxidations of alcohols and sulfides at room temperature using N‐methyl-morpholine‐N‐oxide as oxidant. This provided a general method for the oxidations of different kinds of alcohols under mild conditions. Benzylic primary and secondary alcohols were oxidized to products in excellent yield, and aliphatic and cyclic alcohols gave carbonyl compounds in moderate yield [21].
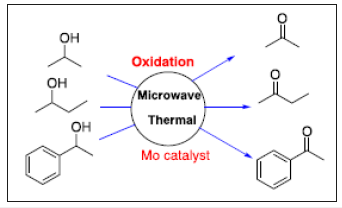
Four di-oxido molybdenum(VI) chelates with ONO tridentate Azomethine ligands derived from 3‐acetyl‐6‐methyl‐(2H)‐pyran‐2,4‐(3H)‐dione (dehydrocetic acid, Hdha) and aromatic hydrazides (benzoyl hydrazide (bhz), isonicotinoyl hydrazide (inh), nicotinoyl hydrazide (nah) and furyl hydrazide (fah)) have been prepared, and then analyzed by elemental analysis, infrared, UV–visible, 1HNMR and 13CNMR spectroscopies and thermogravimetric analyses. The di-oxidomolybdenum (VI) chelates have been studied as catalysts for the homogeneous oxidation of secondary alcohols (1‐phenylethanol, 2‐propanol and 2‐butanol; Figure 1), using 30% H2O2 as an oxidant. Both microwave and liquid‐phase oxidation methods have been tested for catalytic reactions. Also, the various parameters of the reaction, such as amount of catalyst, oxidant, solvent and temperature, have been taken into consideration for the maximum conversion of substrates. Under the optimized reaction conditions, secondary alcohols gave high yields of the respective ketones. Addition of N‐based additive reduced the reaction time and increased the conversion of alcohol [22].
Figure 1:Oxidation of alcohols and sulfides into corresponding aldehydes and sulfoxides.
Oxidation of sulfides to sulfoxides and oxidative coupling of thiols into their corresponding disulfides were carried out using hydrogen peroxide as oxidizing agent in the presence of Ni (II), Co (II), Cr(III), Zn(II) or Cd(II) chelates immobilized on Fe3O4 magnetic nanoparticles (M‐Salen‐MNPs) as stable, heterogeneous, efficient and magnetically recoverable nano catalysts under mild reaction conditions. Also, a variety of aromatic and aliphatic sulfides and thiols with various functional groups were successfully oxidized with short reaction times in good to excellent yields. Recovery of the catalyst is easily achieved by magnetic decantation and it can be reused for several consecutive runs without significant loss of its catalytic efficiency [23,24].
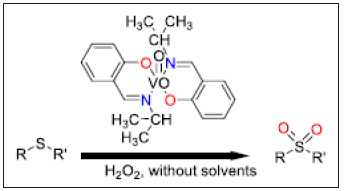
A new bidentate ON azomethine ligand (HL) was prepared by simple condensation reaction of isopropylamine and salicylaldehyde in methanol or chloroform as solvent at ambient temperature. Then, by reaction of HL and VO (acac)2 in a ratio of 2:1 in the presence of triethylamine at room temperature, a new oxovanadium (IV) Azomethine complex, VOL2, was prepared. The catalytic performance of the VOL2 complex was tested in the selective oxidation of thioanisole with the green oxidant H2O2 (35% aqueous) under solvent‐free conditions and with organic solvent (EtOH, CHCl3, CH2Cl2, DMF, CH3CN, EtOAc) as model reaction. Owing to the excellent catalytic performance of the VOL2 complex (Figure 2) under solvent‐free conditions, this complex was used for the oxidation of various sulfides to the corresponding sulfones under solvent‐free conditions. The use of H2O2 as oxidant and the absence of solvent made these reactions interesting from environmental and economic points of view. The amounts of H2O2 played an important role in leading to the selective oxidation of thioanisole into the corresponding sulfone. Also, different sulfides, such as phenyl-alkyl sulfides, di-alkyl sulfides and unsaturated sulfides, were converted into the corresponding sulfones in the presence of VOL2 as catalyst. Sulfide with C=C double bond was selectively oxidized into its corresponding sulfone without affecting reactive functional groups [20]. Cu and Ni nanosized azomethine chelates, namely ahpvCu, ahpnbCu, and ahpvNi, incorporating azomethine ligands derived from the condensation of 2-amino-3-hydroxypyridine, with either 3-methoxysalicylaldehyde (ahpv) or 4-nitrobenzaldehyde (ahpnb), were synthesized using sono chemical approach. The structure and properties of the new ligands and their chelates with Ni (II) and Cu (II) were determined via infrared, nuclear magnetic resonance, electronic spectra, elemental analysis, thermal gravimetric analysis, molar conductivity, and magnetic moment. The combined results revealed the formation of 1:1 (metal: ligand) chelates for ahpvCu and ahpvNi and 1:2 for ahpnb Cu. Additionally, CuO and NiO nanoparticles were prepared by calcination of the respective nanosized Cu/Ni chelates at 500 ̊C and characterized by powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). Significantly, the as prepared nanosized Azomethine Cu/ Ni chelates and their oxides showed remarkable catalytic activity towards the selective oxidation of benzyl alcohol (BzOH) in aqueous H2O2/di-methyl sulfoxide (DMSO) solution. Thus, catalytic oxidation of BzOH to benzaldehyde (BzH) using both ahpvCu complex and CuO nanoparticles in H2O2/DMSO media at 70 ̊C for 2h yielded 94% and 98% BzH, respectively, with 100% selectivity [20].
Figure 2:Oxidation of sulfides to corresponding sulfones under solvent free conditions.
Conclusion
Azomethine ligands are known as an important class of organic compounds because of their capacities to bind metal ions from environmental media as well as being efficient catalysts for oxidation of alkanes and alcohols, epoxidation of alkenes and sulfoxidation of organic compounds in the presence of suitable oxidants, e.g. alkyl hydroperoxides, H2O2 or O2, under mild conditions. The results reviewed here showed that Schiff bases and their complexes exhibit efficient catalytic activity towards the selective epoxidation of simple alkenes, and primary and secondary alcohols. Immobilization on solid supports was one of the desirable strategies for facilitating catalyst separation and recycling. The topics reviewed in this study are industrially promising in the fields of catalysis with multi‐ and interdisciplinary approaches. Although the developments of interest have been made in oxidation catalysis using azomethine complexes as discussed herein, it is necessary to address further the development of green processes. Consequently, the use of H2O2 as a mild oxidant in the presence of the synthesized catalysts would provide an efficient, easy and safe approach, leading to oxidations of alcohols to corresponding compounds. Thus, green (using H2O2), simple, clean and economical procedures for oxidation of various organic compounds is recommended.
References
- Abdel Rahman LH, El-Khatib RM, Nassr LAE, Abu-Dief AM, Lashin FE (2013) Synthesis, physicochemical studies, embryos toxicity and DNA interaction of some new Iron (II) Schiff base amino acid complexes. J Mol Struct 1040: 9-18.
- Abdel Rahman LH, El-Khatib RM, Nassr LAE, Abu-Dief AM, Ismael M, et al. (2014) Metal based pharmacologically active agents: Synthesis, structural characterization, molecular modeling, CT-DNA binding studies and in vitro antimicrobial screening of iron(II) bromosalicylidene amino acid chelates. Spectrochim Acta A Mol Biomol Spectrosc 117: 366-378.
- Abu-Dief AM, Díaz-Torres R, Sañudo EC, Abdel-Rahman LH, Alcalde NA (2013) Novel sandwich triple-decker di-nuclear Nd III-(bis-N,N’ -pbromo-salicylideneamine-1,2-diaminobenzene) complex. Polyhedron 64: 203-208.
- Abu-Dief AM, Nassr LAE (2015) Tailoring, physicochemical characterization, antibacterial and DNA binding mode studies of Cu(II) Schiff bases amino acid bioactive agents incorporating 5-bromo-2-hydroxybenzaldehyde. J Iran Chem Soc 12(6): 943-955.
- El-Lateef Abd HM, Abu-Dief AM, Abdel-Rahman LH, Sañudo EC, AliagaAlcalde N (2015) Electrochemical and theoretical quantum approaches on the inhibition of C1018 carbon steel corrosion in acidic medium containing chloride using some newly synthesized phenolic Schiff bases compounds. J Electroanal Chem 743: 120-133.
- Abdel-Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM (2016) Some new Nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorg Chem 69: 140-152.
- Abdel-Rahman LH, Abu-Dief AM, Aboelez MO, Abdel-Mawgoud Azza AH (2017) DNA interaction, antimicrobial, anticancer activities and molecular docking study of some new VO(II), Cr(III), Mn(II) and Ni(II) mononuclear chelates encompassing quaridentate imine ligand. J Photochem Photobiol B 170: 271-285.
- Abdel-Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM (2018) Sonochemical synthesis, spectroscopic characterization, 3d molecular modeling, DNA binding and antimicrobial evaluation of some transition metal complexes based on bidentate no donor imine ligand. Int J Nano Chem 4: 1-77
- Abdel-Rahman LH, Abu-Dief AM, Shehata Mohamed R, Atlam Faten M, Abdel-Mawgoud Azza AH (2019) Some new Ag(I), VO(II) and Pd(II) chelates incorporating tridentate imine ligand: Design, synthesis, structure elucidation, density functional theory calculations for DNA interaction, antimicrobial and anticancer activities and molecular docking studies. Appl Organometal Chem, p. e4699.
- Abdel-Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM (2016) Sonochemical synthesis, DNA binding, antimicrobial evaluation and in vitro anticancer activity of three new nano-sized Cu(II), Co(II) and Ni(II) chelates based on tri-dentate NOO imine ligands as precursors for metal oxides. J Photochem Photobiol B 162: 298-308.
- Abdel-Rahman LH, Abu-Dief AM, Abdel-Mawgoud Azza AH (2019) Novel Di- and Tri-azomethine compounds as chemo sensors for the detection of various metal ions. Int J Nano Chem 5: 1-17.
- Abdel-Rahman LH, Ismail NM, Ismael M, Abu- Dief AM, Ahmed EA (2017b) Synthesis, characterization, DFT calculations and biological studies of Mn(II), Fe(II), Co(II) and Cd(II) complexes based on a tetradentate ONNO donor Schiff base ligand. J Mol Struct 1134: 851-862.
- Ibrahim EMM, Abdel-Rahman LH, Abu-Dief AM, Elshafaie AH, Samar K, Ahmed AM (2018) The electric and thermoelectric properties of Cu(II)- Schiff base nano-complexes. Physica Scripta 93(5): 055801.
- Abdel-Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM, Adam AM, et al. (2018) Sonochemical synthesis, structural inspection and semiconductor behavior of three new nano sized Cu(II), Co(II) and Ni(II) chelates based on tri-dentate NOO imine ligand as precursors for metal oxides. Appl Organometal Chem 32(3): e4174.
- Elshafaie A, Abdel Rahman LH, Abu Dief AM, Samar KH, Samar K, et al. (2018) Electric, thermoelectric and magnetic properties of Nickel(II) Imine Nanocomplexes, NANO: Brief Reports and Reviews 13(7): 1850074.
- Abu Dief AM, Mohamed, Ibrahim MA (2015) A review on versatile applications of transition metal complexes incorporating Schiff bases. J Basic Appl Sci 4: 119-133.
- Abdel Rahman LH, Abu Dief AM, Adam MSS, Hamdan SK (2016) Some new nano-sized mononuclear Cu(II) schiff base complexes: Design, characterization, molecular modeling and catalytic potentials in benzyl alcohol oxidation. Catal Lett 146: 1373-1396.
- . Abdel Rahman LH, Abu Dief AM, Abdel Mawgoud, Azza AH (2019) Development, structural investigation, DNA binding, antimicrobial screening and anticancer activities of two novel quari-dentate VO(II) and Mn (II) mononuclear complexes. J King Saud Uni 31(1): 52-60.
- Abdel Rahman LH, Abu Dief AM, Basha M, Abdel Mawgoud, Azza AH (2017) Three novel Ni(II), VO(II) and Cr (III) mononuclear complexes encompassing potentially tri-dentate imine ligand: synthesis, structural characterization, DNA interaction, antimicrobial evaluation and anticancer activity. Appl Organometal Chem, p. e3750.
- Al Saeedi, Sameerah I, Abdel Rahman LH, Abu Dief, Abdel Fatah, et al. (2018) Catalytic oxidation of benzyl alcohol using nanosized Cu/Ni schiff-base complexes and their metal oxide nanoparticles. Catalysts 8(452): 1-14
- Ghorbani C, Darvishnejad Z, Norouzi M (2015) Cu (II)-Schiff base complex-functionalized magnetic Fe3O4nanoparticles: A heterogeneous catalyst for various oxidation reactions. Appl Organometal Chem 29(3): 170-175
- Maurya MR, Saini N, Avecilla F (2015) Vanadium complexes derived from acetyl pyrazolone and hydrazides: structure, reactivity, peroxidase mimicry and efficient catalytic activity for the oxidation of 1‐ phenylethanol. Polyhedron 25: 4028-4044.
- Godhani DR, Nakum HD, Parmar DK, Mehta JP, Desai NC (2016) Oxy functionalization of olefins with neat and heterogenized binuclear VO(IV) and Fe(II) complexes: Effect of steric hindrance on product selectivity and output in homogeneous and heterogeneous phase. J Mol Catal A 474: 110424.
- Menati S, Rudbari HA, Korshidifard M, Jalilian F (2016) A new Oxovanadium (IV) complex containing an O, N-bidentate Schiff base ligand: Synthesis at ambient temperature, characterization, crystal structure and catalytic performance in selective oxidation of sulfides to sulfones using H2O2 under solvent-free conditions. J Mol Struct 1103: 94- 102.
© 2019 Abu-Dief AM. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






