- Submissions

Full Text
Annals of Chemical Science Research
Tribological Test and Mechanical Properties of Electrodeposited Nickel Coating on the Metal Surface of Carbon Steel 4140
Alexander NK1, Olga Bunkova2, Frontasyeva MV3*, Pavel Nekhoroshkov3, Alexandra Kravtsova3, Inga Zinicovscaia3 and Nikita Yushin3
1National Institute of Nuclear Research, México
2Faculty of Chemistry, México
3Joint Center for Research in Sustainable Chemistry UAEM-UNAM, México
4SEPI-ESIME Zacatenco, México
*Corresponding author: Torres JV, National Institute of Nuclear Research, México
Submission: May 11 2019;Published: May 28, 2019

ISSN : 2688-8394 Volume1 Issue3
Abstract
In this study, we used the electrodeposition technique of nickel on the metal surface of carbon steel 4140. To produce the coating, we worked with Watts and Sulfamate baths. The sliding wear properties were analyzed for tribological test, so as the roughness, hardness, and thingness layer, all these in accordance with the normative ASTM.
Keywords:Nickel coating; Electrodeposition; Adhesion; Hardness; Corrosion
Introduction
Wear and corrosion are the main problems in mechanical elements in mechanical contact and therefore there are economical losses. To enhance the tribological properties of the metallic materials, nickel coating has found a wide application in aerospace, automobile, chemical industries and other. In this research nickel coating on carbon steel substrate were developed using nickel sulfamate bath and Watts nickel bath to obtain homogeneous electrodeposits so as good adherence and nonporous material. Nickel is one of the most used metals in the nuclear industry as well as in conventional industry, so it is important to study its morphological composition and its mechanical properties. The crystal structure of nickel: It is distributed in two phases, gamma (γ) and gamma prime (γ›): -Gamma phase: solid solution centered on the faces that acts as a matrix. Gamma prime phase: dispersion of ordered intermetallic precipitates, responsible for the great resistance of the super-alloys.
Experimental development
Electroless nickel coatings [1-3] based on the bath types have been progressively applied to a wider variety of applications in Industry. Several researches [4,5] have investigated the application of different electroless nickel coatings. They have observed that the nickel coating improved the tribological properties and protect the material surfaces in mechanical contact. In addition, the coating process can provide properties to reduce the stresses, to improve the adhesion and the corrosion resistance. In this study, we used the electrodepositions technique of nickel to produce a coating on the metallic surface of carbon steel with the objective to reduce the corrosion process. To obtain these coating we worked with two solutions, one of them is, Watts bath and the other one is sulphamate bath [6-10]. We used an electrolytic cell made of Pyrex glass. To produce the electrodeposition on the metallic surface of carbon steel used as cathode, in the upper head of the electrolytic cell was adapted a cap made of Teflon with 3 holes where it was connected whit two anodes in the extremes and one cathode middle of the cap, as anode was used nickel bars. A constant temperature circulator was adapted to the electrolytic cell through a hose. As power supply was used a galvanostat.
Preparation of electrolytes
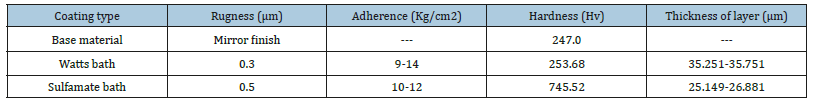
Chemical compounds were chemically pure. These were mixed with 250ml of deionized water using an agitator (80rpm) in order to dissolve completely all the compounds. The electrolytes used to make the different nickel coatings are shown in Table 1. The coatings obtained were analyzed by scanning electronic microscope (SEM JSM6610-LV) obtaining the results shown in Figure 1 and 2. Rugness, Adhesion, hardness and Thickness of layer tests in accordance with the standards ASTM 4138 [11], ASTM D 4541-02 [12], ASTM E140-05 [13], ASTM D4138-94(1999) [14] respectively. The coatings were made with mirror-finished base material to obtain the surface characteristics shown in Table 2. The coatings obtained with the electrodeposition process under specific controlled conditions such as: temperature, current density, exposure time, pH, electrolyte concentration and agitation, produced and uniform layer of nickel over the entire surface.
Figure 1:Percentage of nickel in the samples coated with Watts bath, SEM; 1000x.

Figure 2:Percentage of nickel in the samples coated with nickel sulphamate bath, SEM; 1000x.

Table 1:Components of the Watts bath.
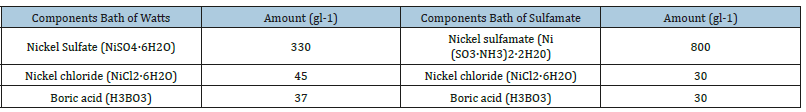
Table 2:Data obtained from mechanical tests.
Tribological tests
Table 3:Results of the sliding tests in accordance a normative ASTM G0099-04a [8].

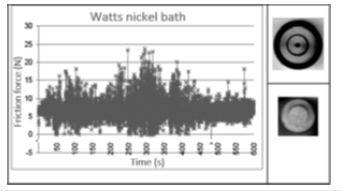
The sliding wear properties have been analyzed for 4140 carbon steel pin specimens uncoated, 4140 carbon steel pin specimens coated with Watt nickel bath, and 4140 carbon steel pin specimens coated with nickel sulfamate bath, using a pin-on-disk tester at constant load of 5 N (≈ 0.2MPa), the test time was 600s, the velocity was 0.95m/s, the rotational speed was 200rpm, and the wear track radius was 0.047m. The tests were carried out at ambient temperature from 22 to 24 °C in dry condition. The material of the disk was stainless steel 304. Table 3 shows the results of sliding length (L), wear volume loss (Vw), wear rate (Q), wear factor (K), friction force, (Ff) and friction coefficient (μ). Figure 3-5 show the friction force as a function of the time. From Figure 3 it can been seen that there are large and irregular fluctuations of the friction force during the sliding test. In addition, it is observed that the friction coefficient of 0.96 is found under the values reported by the literature. The results shown in Figure 5 suggest that the coating with Watts nickel bath protected for a short time, approximately 20seconds, the 4140carbon steel pin specimen surfaces. While the coating nickel sulfamate bath presented more sliding wear resistance, which resists up to 250seconds. Therefore, the friction force and friction coefficient were smaller than the coated pin specimen with the Watts nickel bath and the pin specimen uncoated [9,10].
Figure 3:Friction force of the 4140-carbon steel pin specimen uncoated as function of the time.
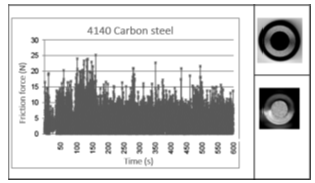
Figure 4:Friction force of the 4140carbon steel pin specimen coated with sulfamate nickel bath as function of the time.

Figure 5:Friction force of the 4140carbon steel pin specimen coated with Watts nickel bath as function of the time.
The comparison of the results obtained from the corrosion test of the 4140carbon steel, the coated carbon steel with Watts nickel bath and coated carbon nickel sulfamate bath are shown in Table 1. From the results it can be observed a high polarization with coating nickel. The coated 4140 carbon steel with nickel sulfamate bath presented the highest resistance to the polarization and therefore the corrosion velocity was the lowest.
In the obtained coatings it can be seen that the mechanical properties are improved with respect to the base material since there is a hardness gain of up to 2.5 times the hardness of the base material, in layers of approximately 25μm. Tribological tests were also carried out to test the hardness of the electrodeposits, the results indicate that the nickel coating obtained by the sulphamate bath method is 2.5 times harder than the base metal [15].
Conclusion
The contribution of this work shows us that there are different treatments that can be applied in materials, whether surface or bulk, the selection of the type of treatment must be according to the type of work that the material must perform. Electrodeposited coatings change the surface properties of the base materials. In the case of a nickel coating on carbon steel, it produces a higher hardness as a surface characteristic. Due to its nickel properties when applied to a metallic material such as carbon steels that tend to oxidize very easily, the nickel coating would help to avoid this type of phenomenon. On the other hand, the layer of the nickel coating gives a good finish to the material, it should be borne in mind that the quality of the electrodeposition, will depend a lot on the surface finish that you have on the base part.
References
- Rymuza Z (1989) Special tribological coatings in tribology of miniature systems. Elsevier, Amsterdam, Netherlands, pp. 269-301.
- Rabinowicz E (1965) Friction and wear of materials. Wiley, New York, USA.
- Bhushan B (2002) Introduction to tribology. Wiley, New York, USA.
- Sahoo P, Das SK (2011) Tribology of electroless nickel coatings-A review. Materials and Design 32(4): 1760-1775.
- Gadhari P, Sahoo P (2014) Study of tribological properties of electroless Ni-P-Al2O3 composite coatings. IOSR Journal of Mechanical and Civil Engineering, pp.34-34.
- Kalpakjian S, Schmid SR (2002) Manufacturing, engineering and technology. (4th edn), Prentice Hall, New Jersey, USA.
- Donald A, Phulé FP (2003) The science and engineering of the materials. Cuarta edition, Tomson Brooks/Cole.
- ASTM G99 (1990) Standard method for wear testing with a Pin-on-Disk. Apparatus.
- Bhushan B (2013) Principles and applications of tribology. (2nd edn), Jhon Wiley & sons inc, New York, USA.
- Reyes Astivia JE, Torres JV, Torres MV (2014) Mechanical properties of carbon steel electroplated with nickel. Tesis, Editorial Académica Española.
- ASTM D 4138 (2007) Dry film thickness of protective coating sistems by destrucive means. ASTM International.
- ASTM D4541-02 (2002) Standard test Method for pull-off strength of coatings using portable adhesion testers. Annual Book of ASTM Standards 6(2).
- ASTM E140-05 (2005) Standard hardness conversion tables for metals relationship among brinell hardness, vickers hardness, rockwell hardness, superficial hardness, knoop hardness, and scleroscope hardness. Annual Book of ASTM Standards 3(1).
- ASTM D4138-94(1999) Standard practices for measurement of dry film thickness of protective coating systems by destructive means. Annual Book of ASTM Standards 6(2).
- Reyes Astivia ЈE (2014) Experimental study of the mechanical and tribological properties of electrodeposited carbon steel with nickel using an electrolytic cell. MSc thesis, SEPI-ESIME Zacatenco, IPN, Mexico.
© 2019 Torres JV. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






