- Submissions

Full Text
Annals of Chemical Science Research
Characterization of Rubber Seed Shell and Kernel (Hevea brasiliensis) as Raw Materials for Coliquefaction with Low Rank Coal
Mohd Azlan Mohd Ishak1*, Siti Nur Ain Mohd Hassan1,2, Ali H Jawad2 and Khudzir Ismail2
1Faculty of Applied Sciences, Universiti Teknologi MARA, Perlis Branch, 02600 Arau, Perlis, Malaysia
2Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
*Corresponding author: Mohd Azlan Mohd Ishak, Faculty of Applied Sciences, Universiti Teknologi MARA, Malaysia
Submission: January 15, 2019;Published: January 22, 2019

ISSN : 2688-8394 Volume1 Issue1
Abstract
The present study focuses on characterization of two biomass samples namely rubber seed shell (RSS) and rubber seed kernel (RSK), as potential raw materials for co-liquefaction with Mukah Balingian low rank Malaysian coal. The physical and chemical characteristics of the biomass samples such as proximate and ultimate analyses, number of extractives, holocellulose and hemicelluloses content, calorific value, bio-oil yield and pyrolysis behavior were determined. Apparently, RSK was found to be more suitable as co-liquefaction material with Mukah Balingian coal due to its high carbon (64.5wt%), high volatile matter (92.4wt%) content, and high percent yield of bio-oil (33.1wt%). Further, the pyrolysis thermogram indicated that RSK contained higher lignified material than RSS. Moreover, RSK exhibits higher calorific value with comparison to RSS and coal.
Keywords:Biomass; Coal; Co-liquefaction; Rubber seed
Introduction
Malaysia is one of the leading producers of natural rubber in the world with 1.3 million ha of area under rubber plantation [1] varying from more than 20 clones of rubber trees [2]. Each hectare can give an approximate amount of 150kg of seeds [3,4]. The rubber tree is widely used as a source of natural rubber known as latex, and its seeds have been found to be rich in bio-oil. The seeds are inedible and are abundance in the country. The used of rubber seed shell as a raw material for the production of activated carbon were investigated by Sun and Jiang [5] and Ekebafe [6]. The biodiesel production from rubber seed oil has been investigated by many researches [2,7-11]. Rubber seed oil was also used in kaolin intercalates [12]. However, none of the studies thus far, reported the physical and chemical characteristics and bio-oil contents in RSS and RSK, in relation to the potential of these materials for co-liquefaction with low-rank coal.
Co-liquefaction of biomass and coal provides an alternative to the production of fuels and chemical feedstock. Co-processing of agricultural and biomass waste with coal by Mohd Hassan et al. [13] showed that the used of biomass type and agricultural waste as agents of co-liquefaction of coal is in general worthy of consideration. Depci et al. [14] observed that the co-processing of coal with biomass materials increases liquefaction yields. Recent study by Paysepar et al. [15], on co-liquefaction of coal and lignin found that the addition of biomass and ethanol-water were beneficial to liquefaction process. However, the used of rubber seed as the biomass feedstock for co-liquefaction with coal is still superficial. The usage of rubber seed could be of great interest as it is abundance, renewable and relatively cheap in comparison to another biomass. Hence, the objective of this study is to determine the physical and chemical characteristics from different parts of rubber seed i.e. RSS and RSK and evaluate the potential of these parts as feedstock for co-liquefaction with low rank coal. The results of this paper can be of beneficial reference for rubber seed agriculture expanding profit.
Methodology
Materials
The materials used were rubber seeds and low-rank coal (Mukah Balingian). Rubber seeds were obtained from a local supplier in Kedah, Malaysia within the harvesting period of 2012 and were stored at room conditions. The parts of seeds were separated into shell (RSS) and kernel (RSK). All of the samples (rubber seed and coal) were pulverised and sieved to a particle size fraction of 212μm-2mm separately
Chemicals
Acetone, sodium acetate, sodium chlorite, sodium hydroxide and hexane were purchased from HmbG. All the chemicals used were industrial reagent grade.
Ultimate analysis
Carbon, hydrogen, nitrogen, and sulphur content of samples were determined by using CHNS-Analyzer (CHNS-932). The sam-ples were weighed around 0.5 to 1.0mg in an aluminium container prior entering the analyser for percentage composition of C, H, N and S analysis and the percentage O was determined by means of difference.
Proximate analysis
The biomass and coal samples were weighed and placed inside drying oven at constant temperature of 105 °C for 1 h. The dried samples were then cooled to room temperature by placing inside desiccators and re-weighed. Moisture content was then calculated using the following formula (1):
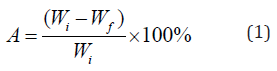
Where A is moisture, Wi is the initial weight of the samples (before drying) and Wf is the weight of the samples post dying.
The ash content was determined in laboratory muffle furnace (Carbolite Type 301) as per ASTM 3174-11 [16]. 1.0g of biomass sample was placed in a crucible and inserted into muffle furnace maintained at 600±10 °C for 4h. Then the crucible was removed from the furnace and placed inside the desiccator. The process of heating and cooling was repeated until constant weight was obtained. The volatile matter in the biomass and coal were determined by the procedure given in ASTM D 3175–11 [17]. The biomass sample (1g) was placed in muffle furnace maintained at 950 ± 10 °C for 7min. The crucible was then removed from the furnace and placed inside the desiccators to cool at room temperature. The volatile matter in the samples was determined by the loss of weight. Results of ash and volatile matter were reported in wet basis.
Determination of extractives
Solvent extraction (60ml acetone for 1g of dried biomass sample) was used, and the temperature was held at 90 °C for 2h. The sample was dried at 105 °C until a constant weight was obtained. The weight difference before and after the extraction was accounted as the amount of extractives.
Holocellulose extraction
The procedure used for preparing holocellulose involved the treatment of the biomass samples (4g) with an acid solution (160cm3 sodium acetate solution) at 75 °C for 5h. Sodium chlorite (4cm3) was added every hour during 4h. The mixture is then cooled down and the residue was filtered and washed with water (1dm3) followed with acetone (15cm3). The residue was dried at room temperature; an aliquot was weighted and dried at 105 °C for the determination of the holocellulose content [18].
Hemicellulose extraction
10mL of 0.5mol/L sodium hydroxide was added to 1g of extractive- free dried biomass, and the temperature was held at 80 °C for 3.5h. The samples were then washed using DI water until the pH value of the solution approach 7 and were dried to a constant weight. The difference between the sample weight before and after this treatment was accounted as hemicelluloses content [19]. The cellulose content however was calculated by subtracting the determined hemicelluloses from the known holocellulose content.
Calorific value
A bomb calorimeter (Leco AC-350) equipped with water bath and oxygen controller was used to obtain the calorific value of the biomass and coal samples.
Extraction of oil
Bio-oil from RSS and RSK were extracted from the 10g of samples by soxhlet extraction using hexane as solvent at 60 °C for 6 hours [5]. All supernatants were collected and evaporated in a rotary evaporator to remove the solvent. The percent of bio-oil yield were calculated as below (2):
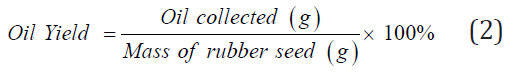
Pyrolysis
The biomass and coal samples were heated under nitrogen atmosphere at temperature range between 30 °C and 700 °C with heating rate of 10 °C/min using thermogravimetric analyser (TGA).
Results and Discussion
Ultimate analysis
Table 1:Ultimate analysis of the biomass samples

*calculated by difference
Table 1 shows the ultimate analysis of the biomass and coal samples. From the analysis, the major components of the samples were carbon and oxygen with hydrogen, nitrogen, and sulphur were present as minor. RSK contained higher percentage of C (64.45%), H (8.23%), N (3.63%) and S (0.32%) with comparison to RSS and coal. The percentages of N and S were less than 4% and 0.5%, respectively and are considered as relatively low. It has been reported that high percentage of N can reduce hydrocarbon yields during thermochemical conversion [20]. Likewise, a high S content can cause sulphation and leads to Cl release which causes corrosion of boilers due to presence of FeCl2 and ZnCl2 [21]. Apparently, the percentage of H in RSS is similar to palm kernel shell (PKS) as reported by Jamaluddin et al. [22] where the PKS was used as co-blending material with coal for combustion.
Proximate composition
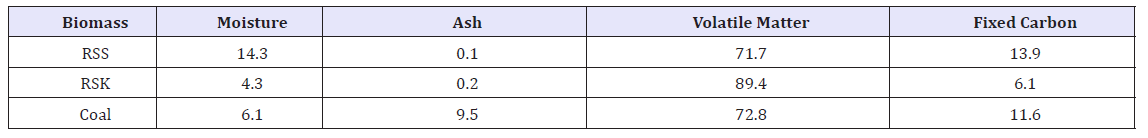
Table 2 represents the proximate analysis for RSS, RSK and coal. RSS contains the highest amount of moisture at 14.2% with RSK the lowest at 4.3%. The moisture content of biomass is an essential operation parameter, which often fluctuates as reported by Hermansson et al. [23]. Moreover, the variation in moisture content caused complication in the operation of the furnaces and results in an uncertainty in the energy content of the fuel delivered to a plant. In terms of ash content, both RSS and RSK exhibit lower percentages of ashes in comparison to coal. A number of biomass that contain similar amount of ash with RSS and RSK are Western Hemlock wood, Mulberry stick, olive pit, coconut fibre, Alabama oak wood waste, sugarcane bagasse, and saw dust [24]. Sasmal et al. [25] reported high ash content retarded the enzymatic hydrolysis i.e. saccharification of biomass samples. However, both RSS and RSK contained low ash and are least to suffer from enzymatic hydrolysis. High amount of volatile matter indicates that the material is easily liquefied. The percent of volatile matter (% dry weight) was much higher in RSK in comparison to RSS and coal. Importantly, both RSS and RSK showed remarkably higher percentages of volatile matter in comparison to Jatropha seed shell, rice husk and pine wood [26].
Table 2:Proximate analysis of the biomass samples
Extractives
Extractives components refer to waxes, fats, resins, gums, sugars, starches, pitch, sterols, flavonoids, tannins, terpenes, quinones, non-structural sugars, chlorophyll and many other minor building block reserves that differ seasonably and according to the biomass type [26]. All these materials have important effect on analysis of structural carbohydrates and lignins. The yields of extractives from the two samples after 2h duration of refluxed showed that RSK contain the lowest amount of extractive with 3.6wt%, with RSS contain the highest amount of extractives at 7.8wt%. Extractives derived from lignocellulosic materials protect plants from termites and other microorganisms. This agrees with the fact that RSS is the outermost layer of the seed that protects the kernel inside the shell.
The knowledge of the number of extractives helps to estimate exact amount of fermentable sugars present in biomass. These substances may be disturbing in pulp and paper mill processes but can also be valuable compounds, which could be utilized, for instance, as raw material in food, pharmaceutical and chemical industry [27]. These materials are chemically polar and non-polar in nature and can be used as green chemicals beside other than just for bio-fuel production. Therefore, these compound needs to be extracted before processing the lignocellulosic biomass as a bio-fuel feedstock [25].Holocellulose extraction
Table 3:Extractives, holocellulose, hemicelluloses and cellulose content of the biomass samples.

*calculated by difference
The content of holocellulose in the samples is presented in Table 3. The first step involves treating the biomass sample with a concentrated acid that disrupts the non-covalent interactions between biomass polymers. The addition of sodium chlorite permits the optimization of the whole polymer hydrolysis while minimizing the degradation of monomeric sugars. It must be pointed out that degradations of sugars are unavoidable during these two steps of hydrolysis [26].
Hemicellulose content
Results obtained from the two different biomass samples differ from each one as presented in Table 3. From Table 3, it shows that RSS has higher amount of hemicelluloses in comparison to RSK, and that of rice straw with value at 43.9wt.% [28]. Hemicelluloses can yield value-added products such as 2,3-butandiol and lactate, as well as bio-ethanol [18]. Therefore, higher hemicelluloses content is better for bio-fuel production as there is useful by-product.
The cellulose contents of RSS and RSK are significantly different as observed in Table 3. According to Kim et al. [28], cellulose con tent of approximately 40% would be useful for bio-fuel production. Apart from single-material bio-fuel production, co-liquefaction of coal and biomass (agricultural/forestry residues and cellulosic waste materials) has gained research interest due to the presence of cellulose in biomass. Cellulose and hemicelluloses in biomass are the keys for liquefaction process as they may be broken down to fuels constituents under direct coal liquefaction conditions [13]. The potential synergistic effect between biomass and coal during liquefaction resulted in enhanced coal conversion and increased in oil yield [29]. Hence, based on these findings, RSK was found to be more suitable and potentially be used as raw material for co-liquefaction with coal.
Calorific value
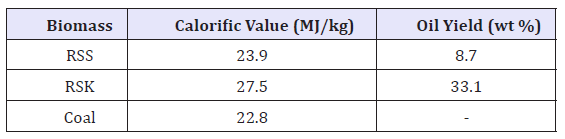
The calorific values of the biomass and coal samples are shown in Table 4. It appears that the heating values of the three samples were in the range of 23.9-27.5MJ/kg. It was observed that RSK showed high calorific value in comparison to RSS and coal. The high calorific value of RSK might be related to its high C content and this indicates the availability of C to form a new shorter bond with H through liquefaction or any other thermo conversion processes. Hence, comparatively, RSK is favourable biomass for the production of bio-fuel.
Table 4:Calorific value and oil yield of the biomass samples.
Bio-oil yield
From Table 4, it reveals that RSK produced the highest bio-oil yield at 33.1wt% with RSS produced lesser bio-oil at 8.7wt%. It has been reported that RSK yield good amount of bio-oil (i.e. between 30 and 40wt%) as reported by Yusup & Khan [4]. Thus, it appears that high C and H contents in RSK have contributed to an increase in the conversion process that resulted to high bio-oil yield being produced. Bio-oil is usually produced through the transesterification of vegetable oil with short-chain alcohols using acids, bases, and enzymes as catalysts. According to the literature, at a certain optimum conditions, the yield of fatty acid methyl esters (FAME) in transesterified RSK oil is in the range of 80.2 to 98.0wt.% [3,8,10]. However, the FAME yield from RSS was not reported by any research due to the low oil yield. Higher cost consumption is needed to collect the oil in terms of the usage of solvent and energy.
Pyrolysis
Figure 1:Thermogravimetric analysis of biomass samples and MB coal.
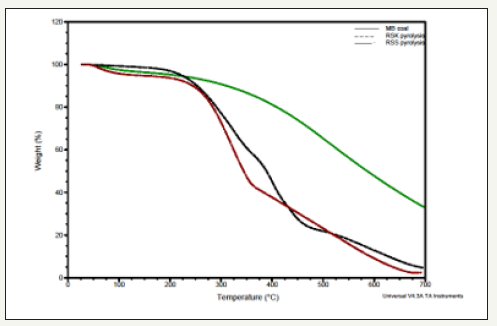
Pyrolysis is the first stage of liquefaction of coal and biomass. The detailed thermal decomposition behaviours of the three samples are illustrated in Figure 1. The initial (Ti) and final (Tf) temperatures of pyrolysis can be determined from TG curves using tangent line method. From Figure 1 it can be observed that Ti of Mukah Balingian coal is at about 355.4 °C and the temperature with the biggest weight loss rate is at 594.2 °C. The Ti of RSS is low, only about 241.7 °C and the temperature of the biggest weight loss rate is 398.0 °C, which are much lower than the corresponding temperatures of Mukah Balingian coal. For RSK, Ti was low with 229.9 °C and after that, the rate of weight loss increased obviously and Tf was 443.6 °C and then became a slow weight loss process above 475.0 °C. The main pyrolysis temperature range (Ti ~ Tf) of Mukah Balingian coal (355.4-594.2 °C) is much higher than that of RSS (241.7-398.0 °C) and RSK (229.9-443. 6 °C) and the releasing rate of volatile matter of Mukah Balingian coal is much lower than that of RSS and RSK. The biggest weight loss rate of RSK is much higher than that of RSS. The results suggest that RSK is very easy to pyrolyze compared to RSS.
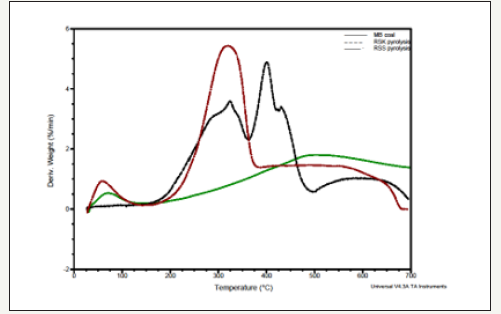
The major degradation of RSS and RSK observed at around 300 °C and 350 °C respectively, which signified the degradation of structural sugar components. Differential thermogravimetric study (DTG) showed two major weight losses for all the biomass samples (Figure 2). The first one was observed within 250-350 °C and the second one was around 400 °C. The value of weight lost at the region between 250 °C and 350 °C signifies the amount of hemicelluloses and cellulose respectively, the lignin degradation started at around 500 °C.
Figure 2:Differential thermogravimetric analysis of biomass samples and MB coal.
Conclusion
The main characteristics of RSS and RSK, cultivated in Malaysia were in order to obtain preliminary data for appropriate selection and suitability for bioconversion. RSK met the requirements of high volatile matter, carbon content and oil yield. From among the two samples, RSK is suggested to be most potential candidate as raw material for co-liquefaction with low rank coal.
Acknowledgement
The authors are grateful to fossil fuel and biomass research laboratory, UiTM Perlis. This work is financially supported by fundamental research grant scheme, FRGS/1/2017/TK10/ UITM/02/11.
References
- Mohammadi M, Man HC, Hassan MA, Yee PL (2010) Treatment of wastewater from rubber industry in Malaysia. African Journal of Biotechnology 9(38): 6233-6243.
- Jose DFM, Raj RE, Prasad BD, Kennedy ZR, Ibrahim AM (2011) A multi-variant approach to optimize process parameters for biodiesel extraction from rubber seed oil. Applied Energy 88(6): 2056-2063.
- Yusup S, Khan M (2010) Basic properties of crude rubber seed oil and crude palm oil blend as a potential feedstock for biodiesel production with enhanced cold flow characteristics. Biomass and Bioenergy 10: 1523-1526.
- Abdullah BM, Salimon J, Yousif E, Salih N (2012) Occurence of cyanogenic glycoside and cyanide in the Malaysian rubber seed oil. Journal of the Association of Arab Universities for Basic and Applied Sciences 14(1): 83-86.
- Sun K, Jiang JC (2010) Preparation and characterization of activated carbon from rubber-seed shell by physical activation with steam. Biomass and Bioenergy 34: 539-544.
- Ekebafe LO, Imanah JE, Okieimen FE (2012) Effect of carbonization on the processing characteristics of rubber seed shell. Arabian Journal of Chemistry 10(1): 174-178.
- Morshed M, Ferdous K, Khan MR, Mazumder MSI, Islam MA, et al. (2011) Rubber seed oil as a potential source for biodiesel production in Bangladesh. Fuel 90: 2981-2986.
- Yang R, Su M, Zhang J, Jin F, Zha C, et al. (2011) Biodiesel production from rubber seed oil using poly (sodium acrylate) supporting NaOH as a water-resistant catalyst. Bioresour Technol 102(3): 2665-2671.
- Ikwuagwu OE, Ononogbu IC, Njoku OU (2000) Production of biodiesel using rubber [Hevea brasiliensis (Kunth. Muell.)] seed oil. Industrial Crop and Products 12(1): 57-62.
- Ramadhas AS, Jayaraj S, Muraleedharan C (2005) Biodiesel production from high FFA rubber seed oil. Fuel 84(4): 335-340.
- Gimbun J, Ali S, Kanwal CCSC, Shah LA, Ghazali NH, et al. (2012) Biodiesel production from rubber seed oil using a limestone-based catalyst. Advances in Materials Physics and Chemistry 2(4B): 138-141.
- Mgbemena CO, Ibekwe NO, Sukumar R, Menon ARR (2013) Characterization of kaolin intercalates of oleochemicals derived from rubber seed (Hevea brasiliensis) and tea seed (Camelia sinensis) oils. Journal of King Saud University-Science 25(2): 149-155.
- Mohd Hassan SNA, Ishak MAM, Ismail K (2017) Optimizing the physical parameters to achieve maximum products from co-liquefaction using response surface methodology. Fuel 207: 102-108.
- Depci T, Karta M, Karaca H (2018) Co-liquefaction process of olive bagasse and peat with lignite and the effect of biomass on the products and oil yield. Energy 156: 750-757.
- Paysepar H, Ren S, Kang S, Shui H, Xu C (2018) Catalytic co-liquefaction of lignin and lignite coal for aromatic liquid fuels and chemicals in mixed solvent of ethanol-water in the presence of a hematite ore. Jour of Anal and App Pyrolysis 134: 301-308.
- ASTM D 3174-11. Standard test method for ash in the analysis sample of coal and coke from coal. Philadelphia.
- ASTM D 3175-11. Standard test method for volatile matter in the analysis sample of coal and coke. Philadelphia.
- Carrier M, Serani AL, Denux D, Lasnier JM, Pichavant FH, et al. (2011) Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass and Bioenergy 35(1): 298-307.
- Lin L, Yan R, Liu Y, Jiang W (2010) In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose and lignin. Bioresour Technol 101(21): 8217- 8223.
- Naik S, Goud VV, Rout PK, Jacobson K, Dalai AK (2010) Characterization of canadian biomass for alternative renewable biofuel. Renewable Energy 35(8): 1624-1631.
- Telmo C, Lousada J, Moreira N (2010) Proximate analysis, backwards stepwise regression between gross calorific value, ultimate and chemical analysis of wood. Bioresour Technol 101(11): 3808-3815.
- Jamaluddin MA, Ismail K, Ishak MAM, Ghani ZA, Abdullah MF, et al. (2013) Microwave-assisted pyrolysis of palm kernel shell: Optimization using response surface methodology (RSM). Renewable Energy 55: 357- 365.
- Hermansson S, Lind F, Thunman H (2011) On-line monitoring of fuel moisture-content in biomass-fired furnaces by measuring relative humidity of the flue gases. Chemical Engineering Reserach and Design 89(11): 2470-2476.
- Shen J, Zhu S, Liu X, Zhang H, Tan J (2010) The prediction of elemental composition of biomass based on proximate analysis. Energy Conversion and Management 51(5): 983-987.
- Sasmal S, Goud VV, Mohanty K (2012) Characterization of biomasses available in the region of North-East India for production of biofuels. Biomass and Bioenergy 45: 212-220.
- Kratzeisen M, Müller J (2013) Suitability of Jatropha seed shells as fuel for small-scale combustion units. Renewable Energy 51: 46-52.
- Puro L, Kallioinen M, Mänttäri M, Nyström M (2011) Evaluation of behavior and fouling potential of wood extractives in ultrafiltration of pulp and paper mill process water. Journal of Membrane Science 368(1- 2): 150-158.
- Kim SJ, Kim MY, Jeong SJ, Jang MS, Chung IM (2012) Analysis of the biomass content of various miscanthus genotypes for biofuel production in Korea. Industrial Crops and Products 38: 46-49.
- Shui H, Cai Z, Chunbao X (2010) Recent advance in direct coal liquefaction. Energies 3: 155-170.
© 2019 Mohd Azlan Mohd Ishak. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






