- Submissions

Full Text
Advancements in Civil Engineering & Technology
Correlation of Apparent Electrical Resistivity with Elastic Moduli and Soil Shear Strength Parameters in Yenagoa, Southern Nigeria
Yusoff MS1,2*, Basenun NFS1, Yusoff NHM1, Aziz HA1,2 and Alazaiza MYD3
1School of Civil Engineering, Universiti Sains Malaysia, Pulau Pinang, Malaysia
2Solid Waste Management Cluster, Science and Technology Research Centre, Universiti Sains Malaysia, Pulau Pinang, Malaysia
3Department of Civil and Environmental Engineering, College of Engineering (COE), A’Sharqiyah University (ASU), Oman
*Corresponding author: Yusoff MS, School of Civil Engineering, Universiti Sains Malaysia suffian@usm.my
Submission: September 08, 2020;Published: October 09, 2020

ISSN: 2639-0574 Volume4 Issue3
Abstract
This paper investigates the removal efficiency of textile wastewater using electrolysis process by carbon electrodes. The performance of COD, turbidity, and color reductions was evaluated under different conditions of pH, voltage usage, and distance between electrodes. The optimum conditions were determined, and the size of the flocs using these conditions was determined. Results showed that maximum COD, turbidity and color reduction were achieved under optimum pH6 conditions, constant 9V voltage, and 1cm distance between the electrodes with an average of 85.5%, 89.2%, and 85.2% respectively. It was found that higher voltage value, shorter distance between electrodes and neutral pH resulted in higher removal efficiency. The flocs size was found to be higher in the case of the short distance between electrodes. Overall, this study demonstrates that the electrolysis process is an effective method of treating textile wastewater and relatively rapid compared to other conventional techniques.
Keywords: Textile wastewater;Electrolysis;Carbon electrode;Flocs size
Introduction
Textile factories generally use high volumes of water and various chemicals for finishing and coloring processes. Wastewater produced from the dyeing process consists of a variety of pollutants, which includes acids, bases, suspended and dissolved solids, toxic and non- biodegradable compounds, which are detected even at low levels and must be treated before the wastewater is released into the environment [1,2]. More than 100,000 commercially available textile colors are estimated to be registered and presented in the industry, where it generates about 700,000-1,000,000 tons of dyes, and approximately 280,000 tons were released annually into the global environment through textile industry effluents [3]. Dyeing and finishing processes are two subsequent stages in the textile manufacturing process. These stages include man-made or natural fibres being tinted to the required permanent color and processed into final commercial items [4]. Dyes can potentially have a harmful effect on human health and can be carcinogenic [5]. The existence of dyes in wastewater, even at low concentrations, is not acceptable as they can lead to esthetic and hazardous impacts on the environment when they are disposed of. In general, textile wastewater encompasses strong colors, high pH fluctuation, and high concentration of COD and suspended solids (SS) [6]. The process of treating this textile wastewater is therefore very difficult because of these properties.
During the last few decades, several researchers have applied different treatment techniques for textile wastewater [7-11]. The selection of a proper treatment technique usually depends on the textile plant’s manufacturing process and chemicals, effluent constituents, discharge requirements, reuse/recycling options and the skills and expertise available. In some textile mills, the effluent produced from various processes can be distinguished as concentrated (paddler-tinting/finishing fillings, printing and dye-baths), medium polluted (washing and rinsing) and low/zero polluted wastewater [12]. This effluent separation assists in the selection of efficient treatment methods. However, in most cases, effluents produced from different processes are discharged from the final outlet together as composite wastewater [13].
Textile wastewater treatment processes can be divided into three known processes: separation and concentration processes, processes of decomposition and degradation, and process of exchange [14]. Usually, a proper combination of these processes is applied in full-scale treatment systems to meet the final discharge according to water treatment standards. Anaerobic biodegradation (as a pretreatment technique) is generally integrated into the treatment of textile wastewater that has high- resistance effluents [9]. However, anaerobic systems are costly. Several researchers have previously used other methods of treatment for textile wastewater, such as electrochemical [15,16], adsorption [17], advanced oxidation [18] and coagulation [19]. Coagulation-flocculation technique is a cheap and cost-effective method, easy to operate, and does not requires high energy. However, the efficiency of the coagulation process is not high for soluble dyes [19]. In addition, the coagulation process generates a high amount of sludge which can adversely affect the treatment process and increase the cost of treatment [20].
The electrolysis process is widely used for treating wastewater. The method depends on the generation of coagulants in the wastewater through the electrodes, usually made of metals like aluminum or iron. The electrodes release ions which neutralize particle charges and thus initiate coagulation. These ions are able to remove contaminants by merging materials and by electro-flotation [21]. The main processes were electrolytic reactions on the surface of the electrode, followed by the formation of coagulants in an aqueous phase, the adsorption of soluble or colloidal pollutants and the removal of pollutants by sedimentation and flotation. The destabilized particles then combine to form flocs, while tiny cathode-formed hydrogen bubbles induce the majority of flocs to help isolate wastewater particles effectively [21].
The main aim of this study is to investigate the effectiveness of color, turbidity and COD removal from textile wastewater using the electrolysis process. Specifically, two electrodes made of carbon were used as electrodes. Different conditions of pH, voltage usage, and distances between electrodes were applied to investigate the optimum conditions that resulted in the maximum removal efficiency. In addition, the size of the flocs was investigated using the optimum conditions determined from experiments.
Materials and Methods
Textile wastewater collection and sampling
Samples of textile wastewater were collected from the Craft Batik Teluk Bahang factory located at Pulau Pinang, Malaysia, in February 2019. The samples were taken directly from a pipe located after dying and washing process using 20L high-density polyethylene bottles to prevent the contamination of samples. The wastewater from the textile process started after dying and washing process in the industry. Normally, in Batik industry, different types of dyes in batik processing were used such as azo dye. Effluents from textile mills were characterized by a high color concentration due to residual dyes. The collected samples were transported to the laboratory within 1hour and kept in a cold room at 4 ℃ to avoid possible chemical and biological reactions before starting the experiments. The samples were acclimatized for 2-3 hours at room temperature before each experiment began. The major parameters tested were pH, turbidity, COD, and color and analysed according to the Standard Method of Water and Wastewater. The main parameters tested were pH, turbidity, COD and color and analysed by the American Public Health Association [22]. The temperature and pH were determined by YSI Professional Plus Quatro, Eutech pH700. Turbidity was determined using a turbidity meter (HACH 2100N). Measurement of COD and colour was done by HACH DR2800 spectrophotometer.
Experimental setup
The treatment of textile effluent using electrolysis process was carried out using different components such as reactor, a pair of electrodes, electrode clamps, direct current (DC) power supply, magnetic stirrer, and magnetic bar. A 500mL glass beaker was used as an electrolytic cell where 300mL of textile sample was used for the test. Two electrodes made of carbon were used in this study. This type of electrode was employed for both cathode and anode electrodes. Carbon was chosen due to cheaper cost and easier to obtain. The electrodes were placed in a glass beaker known as an electrolytic cell. The two electrodes were set up in a vertical position with different distances of 1cm, 2cm, 3cm and 4cm between the cathode and the anode. The electrodes were attached with an electrode clamp in order to ensure that the electrode is in a fixed position when immersed in a textile wastewater sample. The various distances between the electrodes are one of the variables that investigated in this study. Both electrodes were connected to a DC power supply, which supplies electrical current through the electrolysis process. A magnetic stirrer and magnetic bar were also used to enhance pollutant removal [23]. The experiments were performed with different voltages of 3V, 6V, and 9V. In addition, different pH ranges were used in the experiments varied from pH4, pH6, and pH8. All experiments were carried with four different inter distance between electrodes of 1cm, 2cm, 3cm, and 4cm. Three main factors were considered during the experiments like the influence of voltages, the effect of pH, and the impact of distance of inter electrodes. All textile samples were tested for COD, color, and turbidity.
Determination of floc size
Destabilized particles in textile wastewater will aggregate into flocs. Also, cathode will generate tiny hydrogen bubbles and induce the flotation of the majority of flocs, where only a small percentage of the flocs will sink to the bottom. These flocs formed will help to effectively separate particles from wastewater. The size of flocs formed is one of the floc properties; thus, it is significant to measure the size of flocs to know the influence of the size of the flocs on the effectiveness of removal pollutant. In this study, the size of the flocs was measured using the Malvern Mastersizer 2000 particle size analyzer.
Results and Discussion
Characteristics of textile wastewater
The detailed features of the wastewater samples are summarized in Table 1. The raw textile wastewater has a dark-purple color (645Pt Co) with an average pH of 10.98. The acceptable range of color is 200Pt Co. The high pH value indicates that the textile wastewater was alkaline. The acceptable range of pH is between 5.5 and 9 (MQA, 2009). Having high alkalinity is intolerable as it is an indication that the textile wastewaters have the capacity to neutralize acids [24]. On the other hand, color is often caused by forms of organic matter content that cause color pollution induces synthetic chemical dyes and natural dissolved organics such as lignin and tannins [25]. The color and pH exceeded the acceptable conditions for the discharge of industrial wastewater effluent [26]. The COD value was 460mg/L, which is also unacceptable to discharge without treatment (acceptable range for COD is 80-250mg/L). Turbidity value was 24.22 NTU which is also higher than the acceptable range for a discharge without treatment.
Table 1: Textile wastewater characteristics.

Color removal
As stated, different pH was used to determine the optimum pH of the treatment. Figure 1 portrays the removal efficiency of color using different pH, different voltages, and different distances between electrodes. Using raw pH of textile wastewater (10.25) and 9V, the removal efficiency of color at distance of inter-electrode of 1cm, 2cm, 3cm and 4cm was 43.9% (562 to 315PtCo), 26.3 % (562 to 414PtCo), 25% (415 to 313PtCo) and 23% (418 to 319PtCo), respectively (Figure 1). It can be noted that the maximum removal of color was obtained at 1cm of a distance of inter-electrode (43.9%), whereas the lowest removal of color was obtained at 4cm distance of inter-electrode (23%). When the voltage was changed to 6V, the reduction of color was 25.3%, 23%, 21%, and 18% at a distance of inter-electrode of 1cm, 2cm, 3cm and 4cm, respectively. When the voltage value was decreased to 3V, the removal efficiency of color was 29%, 28%, 25% and 23% at the different distance of inter-electrodes. In conclusion, the highest color reduction was achieved using 9V at an inter-electrode distance of 1 cm with 43.9% (562 to 398PtCo).
At pH4 of electrolysis (Figure 1) and using a voltage value of 9V, the percentage removal of color was 63% (463 to 169PtCo), 52% (463 to 220PtCo), 39% (305 to 186PtCo), and 35% (315 to 203PtCo) at the different distance of inter-electrodes. When the constant voltage decreased to 6V, the color reduction was 30.1%, 29%, 28% and 26%. When the constant voltage was decreased again to 3V, the percentage removal of color was 27.8%, 21%, 19% and 13% at a distance of inter-electrode of 1cm, 2cm, 3cm and 4cm respectively. According to Manikandan et al. [27], the color removal at pH4 was 69.31% which is slightly higher than the maximum color removal obtained from the current study (63%) at 1cm distance of inter-electrode and 9V. In general, by using low pH, a higher volume of hydrogen is produced by electro-reduction in the cathode; therefore, less proportion of hydroxide ions can result. By comparing the results of pH6, the acidic condition resulted in the least efficiency than neutral condition. This is because, during the electrolysis process, the dominant carbon species will act as a coagulating agent. At pH4, the removal efficiency was lower because of the formation of soluble carbon complexes [28].
At pH 6 (Figure 1) and using constant voltage of 9V, the percentage removal of color was 85.2% (687 to 102PtCo), 81% (468 to 82PtCo), 69% (353 to 109PtCo), and 65% (350 to 122PtCo) at the different distance of inter-electrodes. When the constant voltage was decreased to 6V, the removal efficiency of color was decreased to 63.4%, 61%, 54%, and 50% using the previous distances between electrodes, respectively. With more decreasing of constant voltage to 3V, the removal efficiency was decreased to 52.3%, 51%, 46%, and 41% at the same distances between electrodes. According to Naje et al. [29], the color removal using pH6 and distance of inter-electrode 1cm was 94% which is slightly higher than the result obtained from the current study (85.2%). Chen [30] stated that higher voltages lead to higher removal.
When the pH of the electrolysis was increased to 8 (Figure 1), and a constant voltage of 9V, the removal efficiency of color was 69.8% (891 to 269PtCo), 43% (497 to 280PtCo), 36% (390 to 249PtCo), and 32% (388 to 265PtCo) at the same distances between electrodes. However, when the value of voltage was decreased to 6V, the removal efficiency of color decreased to 30.2%, 29%, 27% and 23% at the same distances between electrodes. Further reduction in voltage to 3V resulted in a reduction of color removal efficiency to 26.3%, 24%, 21% and 18% at the same distances between electrodes. Overall, it can be summarized that maximum color reduction was achieved at pH 6, 9V constant voltage and 1cm distance between the electrodes.
Figure 1: Effect Removal efficiency of color versus distance between inner electrodes at (a) raw pH, (b) pH 4, (c) pH 6, and (d) pH 8

Turbidity removal
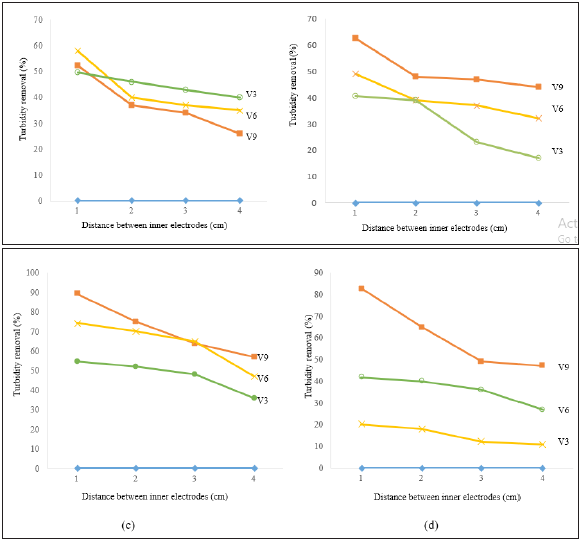
Similarly, the turbidity removal of textile wastewater was evaluated using different pH values, constant voltage values, and distances between electrodes as shown in Figure 2. Using raw pH of electrolysis (Figure 2) and voltage value of 9V, the removal efficiency of turbidity was 52.3% (24.44 to 11.65NTU), 37% (21.07 to 13.28NTU), 34% (22.8 to 15.4NTU), and 26% (22.1 to 16.3NTU) at distances between electrodes of 1cm, 2cm, 3cm and 4cm, respectively. In contrast to color removal, when the voltage value decreased to 6V, the removal efficiency of turbidity was increased to 58%, 40%, 37%, and 35% at the same distances as mentioned earlier. Further decrease of voltage value to 3V leads to higher removal efficiency of turbidity using the same distances between electrodes except for the case of 1cm distance between electrodes. The percentage removal of turbidity was 49.6%, 46%, 43%, and 40%.
When the pH was increased to 4 (Figure 2), and a voltage value of 9V, the percentage removal of turbidity was 62.6% (7.84 to 2.93NTU), 48% (23.83 to 12.26NTU), 47% (33.46 to 17.5NTU), and 44% (32.8 to 18.3NTU) using the different distances between the electrodes. When the voltage value was decreased to 6V, further decrease in turbidity was noted and reached to 49.1%, 39%, 37% and 32%. Further decrease in the voltage value to 3V resulted in lower removal efficiency of turbidity of 40.5%, 39%, 23% and 17% at the different distances between electrodes.
When the pH was increased to 6 (Figure 2), and a voltage value of 9V, the percentage removal of turbidity was 89.2% (10.08 to 1.08NTU), 75% (35.88 to 8.96 NTU), 64% (20.92 to 7.55 NTU), and 57% (22.6 to 9.63 NTU) at the different distances between electrodes. The removal efficiency of the turbidity at pH6 is obviously higher than that of the raw pH and pH of 4. According to Zarei et al. [31], the acidic condition will result in slightly lower removal of turbidity than neutral condition. This finding is due to the formation of small flocs that impact the coagulation and sedimentation process and reduce its effectiveness. When the voltage values decreased to 6V and 3V, the removal efficiency of turbidity was decreased to 74.3%, 70%, 65%, 47% and 54.7%, 52%, 48% and 36% respectively.
At pH 8 (Figure 2) and using voltage values of 9V, the percentage removal efficiency of turbidity was 82.8% (12.15 to 2.08 NTU), 65% (32.51 to 11.5 NTU), 49% (32.48 to 16.64 NTU), and 47% (30.5 to 16.1 NTU). Further decrease of voltage value to 6V and 3V resulted in lower removal efficiency of turbidity to 20%, 18%, 12%, 11% and 41.8%, 40%, 36%, and 27%, respectively. It can be summarized that at optimum conditions pH 6, 9V voltage value and 1cm distance between electrodes, the highest removal efficiency of turbidity was achieved.
Figure 2: Removal efficiency of turbidity versus distance between inner electrodes at (a) raw pH, (b) pH4, (c) pH6 and (d) pH8
COD removal
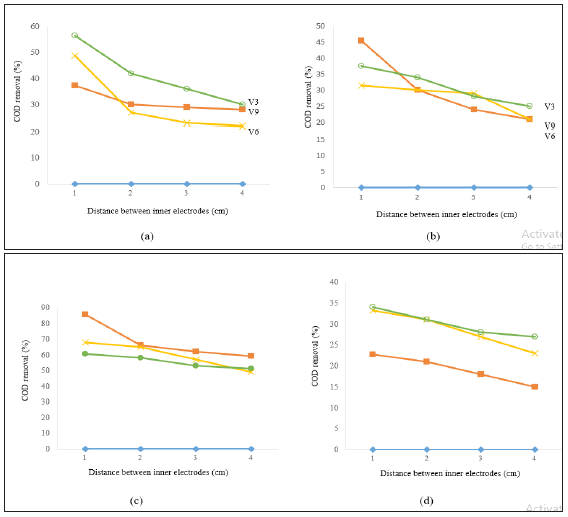
COD removal was also observed under different conditions of pH, voltage value, and distances between electrodes, as shown in Figure 3. At raw pH (Figure 3) and using 9V as a constant voltage, the removal efficiency of COD was 37.2% (456 to 286mg/L), 30% (415 to 290mg/L), 29% (412 to 293mg/L), and 28% (422 to 303mg/L) at inner distances between electrodes of 1cm, 2cm, 3cm and 4cm, respectively. On the other hand, at constant voltage value of 6V, the removal efficiency of COD was 48.7%, 27%, 23% and 22% at the different distances between electrodes. However, when the voltage decreased to 3V, the COD reduction increased to 56.5%, 42%, 36% and 30% at the different distances between electrodes. It is obvious that variation in voltage value plays a central role in the process of treatment. The discussion of the influencing parameters will be discussed in the next section.
Using pH of 4 (Figure 3) and voltage value of 9V, the removal efficiency of COD was 45.4% (460 to 251mg/L), 30% (410 to 284mg/L), 24% (331 to 250mg/L), and 21% (323 to 254mg/L) at the different distances between electrodes. When the voltage value was decreased to 6V and 3V, no significant removal of COD was observed where the maximum removal efficiency was 31.5%, 30%, 29%, 21% and 37.6%, 34%, 28% and 25%, respectively. When pH was increased to 6 (Figure 3), and using a voltage value of 9V, the percentage removal of COD was 85.5% (460 to 66mg/L), 66% (643 to 216mg/L), 62% (447 to 166mg/L), and 59% (440 to 178mg/L). However, when the voltage value was decreased to 6V and 3V, the removal efficiency of COD was decreased to 67.9%, 65%, 57%, 49% and 60.5%, 58%, 53%, 51%, respectively.
Further increase in pH to 8 (Figure 3) resulted in lower removal efficiency of COD of 22.7% (590 to 456mg/L), 21% (442 to 350mg/L), 18% (500 to 412mg/L) and 15% (480 to 410mg/L) at the different distances between electrodes. However, when the voltage decreased to 6V and 3V, a small increase in the performance of COD was noted where the higher removal efficiency was 33.2%, 31%, 27%, 23% and 34.08%, 31%, 28%, 27%, respectively. Naje et al. [29] showed that the COD reduction at pH 4 was 77%, whereas in the present study the higher percentage of COD removal at pH 4 was 45.4%, which is slightly different from COD reduction from the previous study. The higher COD removal is most likely due to the larger surface area of flocs that will contribute to quick absorption trap by colloidal particles. In the current study, however, COD removal was lower with the acidic condition. This observation could be due to the types of organic compounds included in textile wastewater samples. From the previous results, it can be summarized that the optimum conditions which gave the maximum removal efficiency of COD were at a pH of 6, 9V of constant voltage, and 1cm between electrodes.
Figure 3: Removal efficiency of COD versus distance between inner electrodes at (a) raw pH, (b) pH4, (c) pH6, and (d) pH8.
Influence of removal efficiency by operating parameters
The removal efficiency of COD, turbidity, and color was investigated under different conditions of pH, voltage values, and distances between electrodes. The three parameters were considered as the influential factors that affect the removal performance. According to Amour et al. [32], the current density applied was the main operating variable for the coagulant production rate, oxygen evolution, heat generation and bubble production rate; thus, it controls the electrolysis process performance as well as operating costs. Voltage is also a significant variable which controls the pollutant removal reaction rate by determining the producing of bubble rate, bubble size and the formation of the floc, which can influence pollutant removal performance by the electro-coagulation process. The distance between electrodes is also an influential factor in the process of electrolysis. According to Azarian et al. [33] the electrodes distancing influences electrolysis energy consumption, particularly when the electrical conductivity of the samples tested is low. In the current study, four different distances have been tested, as mentioned earlier. The distance of 1cm resulted in optimum removal, which was in agreement with Naje et al. [29]. By increasing the distance, the attraction of the dissolved solution between the electrodes and current ions would be reduced. As a result, the flocculation process will be slow and results in decreasing the efficiency of the treatment process. The interaction of colloidal particles or flocs can have a major impact on their settling and floating characteristics if the inter-electrode distance is small (1cm), resulting in high particle collisions and high pollutant removal. Therefore, increasing the inter-electrode distance reduces pollutant removal and reduces the efficiency of the electrolysis process [34].
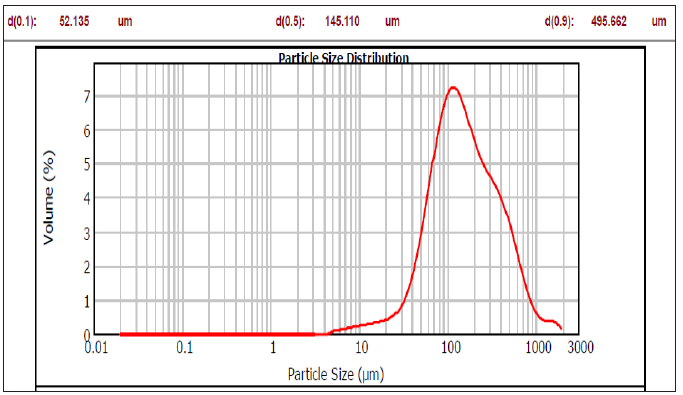
Figure 4: Particle size distribution for flocs at 9V, 1cm between electrodes, and pH6.

Figure 5: Particle size distribution for flocs at 9V, 2cm between electrodes, and pH6.
In addition to the parameters, pH plays a central role in the electrolysis process. According to Atashzaban et al. [35], pH influences the reductions of COD and TSS. The maximum reduction was achieved at both electrodes at a neutral pH of 6-7. Hydrogen bubbles were usually created at neutral pH at the cathode and can be applied to generate an area to link liquid, gas, and solid, and to collect the neutral and colloidal particles. In the current study, the highest removal of pollutant occurred at pH 6 (neutral) condition. During electro-coagulation, the metallic cations created to interact with OH-to form hydroxides which adsorb pollutants (bridge coagulation). In some situations, the hydroxides form larger lattice-like structures that sweep through the water (sweeping coagulation). By neutralizing the colloidal particle charge, cations or hydroxyl ions can also form a pollutant precipitate. In addition, the adhesion of bubbles to flocs results in electro-flotation, which can also help in the removal of pollutants [36]. The interaction between the particles would also cause dense flocs to form. The dense flocs have a large surface area in which soluble organic compounds and colloidal particle trapping are quickly adsorbed, resulting in maximum COD, turbidity and color reduction through flotation and sedimentation.
Size of flocs at optimum conditions
As illustrated earlier, the maximum reduction of the pollutant was obtained at pH of 6, 9V of constant voltage, and 1cm between electrodes. The flocs size was investigated using these optimum conditions, as shown in Figure 4 below. It can be seen that the size of flocs formed at optimum condition was achieved at diameter 0.9 (582.230µm). At this diameter, the largest size of flocs was obtained, which cause high removal of pollutants. This observation is most likely due to the neutralization state, where the flocs start to form as particles collisions occurred. The higher collision efficiency will give a higher removal efficiency of pollutants [37]. When the distance between electrodes increased to 2cm, the flocs were found to be smaller in diameter 0.9 with a value of 495,662μm, as shown in Figure 5. Further increase in the distance between electrodes resulted in smaller flocs size at diameter 0.9 with a value of 252.560µm and 148.392µm for a distance between electrodes of 3cm and 4cm, respectively. From the previous results, it was obvious that the distance between electrodes was an influential factor that affects the size of the flocs. It is believed that the distance between electrodes impacts electrolysis energy consumption, particularly when samples have low electrical conductivity. Increasing the distance between electrodes will reduce the attraction in a dissolved solution between the electrodes and the ions present. Therefore, the size of the flocs will be reduced and hence, the flocculation process will be slow and results. At a closer inter-electrode distance (1cm), colloidal particles or flocs may interact significantly with their settling and flotation characteristics, resulting in high particle collisions, resulting in higher flocs size [34].
Conclusion
In this study, the removal of COD, turbidity, and color from textile wastewater (from the last process of dyeing and finishing) was investigated using the electrolysis process by employing carbon bars as electrodes. Carbon electrodes is found to be an effective coagulant as it generate coagulants in the textile wastewater through the electrodes. Usage of carbon electrode is found to be safe as its not giving detrimental impact towards human health by metal precipitation along the pollutant removal. Electrolysis process was examined under different conditions. It was found that the voltage value affects the rate of reaction of pollutant removal and can affect the removal where the higher voltage value resulted in higher removal efficiency. Also, the distance between electrodes and pH significantly affects the removal efficiency, where the less distance between electrodes resulted in higher removal efficiency and pH plays a central role in the treatment process, where the higher removal efficiency was achieved at neutral pH respectively. Under optimum conditions (pH: 6, constant voltage: 9V, inter-electrode distance: 1cm), 85.5% of COD removal, 89.2% of turbidity removal and 85.2% of color removal were obtained. The flocs size was found to be bigger in the case of less distance between electrodes with 1 cm inter-electrode distance give 582.230μm flocs diameter size. The study demonstrated that the electrolysis process was efficient and relatively rapid and could be used as an alternative treatment for textile effluent in removing COD, turbidity and color from textile wastewater. At present, there is little pollutant removal efficiency in textile wastewater by using carbon electrodes electrolysis was determined. This leaves room for more experimental work to be conducted in order to establish more data in term of other pollutant removal such as suspended solids, heavy metal and phosphate removal to enhance better water quality after electrolysis process.
Acknowledgement
This work was funded by Ministry of Higher Education Malaysia under Fundamental Research Scheme (FRGS) (Grant No. 203/PAWAM/6071415) for research associated with the Solid Waste Management Cluster, Engineering Campus, Universiti Sains Malaysia.
References
- Ballaa W, Essadki AH, Gourich B, Dassaa A, Chenik H, et al. (2010) Electrocoagulation/electro-flotation of reactive, disperse and mixture dyes in an external-loop airlift reactor. J Hazard Mater 184(1-3): 710-716.
- El-Ashtoukhy ESZ, Amin NK (2010) Removal of acid green dye 50 from wastewater by anodic oxidation and electrocoagulation-A comparative study. J Hazard Mater 79(1-3): 113-119.
- Ali H (2010) Biodegradation of synthetic dyes-A review. Water Air Soil Pollut 213: 251-273.
- Khattab TA, Abdelrahman MS, Rehan M (2020) Textile dyeing industry: environmental impacts and remediation. Environ Sci Pollut Res Int27(4): 3803-3818.
- Katheresan V, Kansedo J, Yon Lau S (2018) Efficiency of various recent wastewater dye removal methods: A review. J Environ Chem Eng 6(4): 4676-4697.
- Bakar NA, Othman N, Yunus ZM, Daud Z, Norisman NS, et al. (2020) Physico-Chemical Water Quality Parameters Analysis on Textile. IOP Conference Series: Earth and Environmental Science 498(1): 012077.
- Tian Q, Ran M, Fang G, Ding L, Pan A, et al. (2020) ZnAl2O4/BiPO4 composites as a heterogeneous catalyst for photo-Fenton treatment of textile and pulping wastewater. Separation and Purification Technology 239: 116574.
- Bilińska L, Blus, K, Gmurek M, Ledakowicz S (2019) Coupling of electro-coagulation and ozone treatment for textile wastewater reuse. Chemical Engineering Journal 358: 992-1001.
- Jegatheesan V, Pramanik BK, Chen J, Navaratna D, Chang CY, et al. (2016) Treatment of textile wastewater with membrane bioreactor: a critical review. Bioresource Technology 204: 202-212.
- Naumczyk,J, Szpyrkowicz L, Zilio Grandi F (1996) Electrochemical treatment of textile wastewater. Water Science and Technology 34(11): 17-24.
- Lin SH, Peng CF (1994) Treatment of textile wastewater by electrochemical method. Water Research 28(2): 277-282.
- Paździor K, Bilińska L, Ledakowicz S (2019) A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chemical Engineering Journal 376: 120597.
- Cinperi NC, Ozturk E, Yigit NO, Kitis M (2019) Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. Journal of Cleaner Production 223: 837-848.
- Bechtold T, Mahmud-Ali A, Ganglberger E, Geissler S (2008) Efficient processing of raw material defines the ecological position of natural dyes in textile production. International Journal of Environment and Waste Management 2(3): 215-232.
- Aquino JM, Rocha Filho RC, Bocchi N, Biaggio SR (2013) Electrochemical degradation of the Disperse Orange 29 dye on a β-PbO2 anode assessed by the response surface methodology. J Environ Chem Eng 1(4): 954-961.
- Palácio SM, Espinoza Quiñones FR, Módenes AN, Oliveira CC, Borba FH, et al. (2009) Toxicity assessment from electro-coagulation treated-textile dye wastewaters by bioassays. J Hazard Mater 172(1): 330-337.
- Cao JS, Lin JX, Fang F, Zhang MT, Hu ZR (2014) A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies. Bioresour Technol 163: 199-205.
- Palácio SM, Espinoza Quiñones FR, Módenes AN, Manenti DR, Oliveira CC, et al. (2012) Optimized photocatalytic degradation of a mixture of azo dyes using a TiO2/H2O2/UV process. Water Sci Technol 65(8): 1392-1398.
- Zhao L, Zhang S, Uluko H, Liu L, Lu J, et al. (2014) Effect of ultrasound pretreatment on rennet-induced coagulation properties of goat’s milk. Food Chemistry 165: 167-174.
- Bazrafshan E, Mahvi AH (2014) Textile wastewater treatment by electro-coagulation process using aluminum electrodes. Iranian Journal of Health Sciences 2(1): 16-29.
- Chopra AK, Sharma AK, Kumar V (2011) Overview of electrolytic treatment: An alternative technology for purification of wastewater. Archives of Applied Science Research 3: 191-206.
- APHA (2005) WEF (American public health association, American water works association and Water environment federation). Standard Methods for the Examination of Water and Wastewater.
- Abdullah TA, Salman A, Juzsakova T, Al-Asadi M, Domokos E, et al. (2018) Treatment of oily wastewater using electro-coagulation method with iron poles pers center. In their articles presented, in oral or poster sessions, during the GLOREP 2018 conference, organized in Timisoara, Romania, by 15-17th November 2018, under the auspices of the Balkan Environmental, p. 5.
- Uwidia IE and Ejeomo C (2014) Characterization of textile wastewater discharges in Nigeria and its pollution implications. Global Journal of Research in Engineering.
- Helmy Q, Notodarmojo S, Aruan IA, Apriliawati R (2017) Removal of color and chemical oxygen demand from textile wastewater using advanced oxidation process (AOPs). IPTEK Journal of Proceedings Series 3: 474-481.
- MQA (2009) Environmental quality act 1974, Industrial effluent regulations.
- Manikandan S, Saraswathi R, Ansari AMS (2018) Effect of pH and electrolysis time on removal of reactive black B dye by electrochemical treatment. Asian Journal of Engineering and Applied Technology 7: 45-47.
- Holt PK, Barton GW, Wark M, Mitchell CA (2002) A quantitative comparison between chemical dosing and electrocoagulation. Collision Surface Part A: Physicochemical Engineering 211(2-3): 233-248.
- Naje AS, Chelliapan S, Zakaria Z, Abbas SA (2015) Treatment performance of textile wastewater using electro-coagulation (EC) process under combined electrical connection of electrodes. Int J Electrochem Sci 10: 5924-5941.
- Chen G (2004) Electrochemical technologies in wastewater treatment. Separation and Purification Technology 38(1): 11-41.
- Zarei A, Biglari H, Mobini M, Dargahi A, Ebrahimzadeh G, et al. (2018) Disinfecting poultry slaughterhouse wastewater using copper electrodes in the electro-coagulation process. Polish Journal of Environmental Studies 27(4): 1907-1912.
- Amour A, Merzouk B, Leclerc JP, Lapicque F (2016) Removal of reactive textile dye from aqueous solutions by electro-coagulation in a continuous cell. Desalination and Water Treatment 57(48-49): 22764-22773.
- Azarian G, Rahmani AR, Atashzaban Z, Nematollahi D (2018) New batch electro-coagulation process for treatment and recovery of high organic load and low volume egg processing industry wastewater. Process Safety and Environmental Protection 119: 96-103.
- Sridhara R, Sivakumar V, Immanuel V, Maran JP (2011) Treatment of pulp and paper industry bleaching effluent by electro-coagulant process. Journal of Hazardous Material 186(2-3): 1495-1502.
- Atashzaban Z, Seidmohammadi A, Nematollahi D, Azarian G, Shayesteh OH, et al. (2016) The efficiency of electrocoagulation and electro-flotation processes for removal of polyvinyl acetate from synthetic effluent. Avicenna Journal of Environmental Health Engineering 3: 7469-7469.
- Holt PK, Barton WL, Mitchell CA (2005) The future for electrocoagulation as localized water treatment technology. Chemosphere 59(3): 355-367.
- Yukselen MA, Gregory J (2004) The reversibility of floc breakage. International Journal of Mineral Processing 73(2-4): 251-259.
© 2020 Yusoff MS. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






