- Submissions

Full Text
Advancements in Civil Engineering & Technology
The Conversion of Magnesium Carbonates into Plaster-Like Products: A Preliminary Study of the Hardening Mechanism
Yousef M Alhorr1, Ammar Elhoweris1*, Jennie Morrison2 and José Luis Gálvez Martos3
1 Gulf Organisation for Research and Development, Qatar
2 Department of Chemistry, Meston Building, University of Aberdeen, UK
3 Systems Analysis Unit, IMDEA Energy, Spain
*Corresponding author: Ammar Elhoweris, Gulf Organisation for Research and Development, QSTP, Tech 1, Level 2, Suite 203, PO Box: 210162, Doha, Qatar
Submission: August 15, 2018;Published: August 17, 2018

ISSN: 2639-0574 Volume1 Issue5
Abstract
Magnesium carbonate trihydrate, nesquehonite, is proposed as a precursor for the production of a construction material similar to plasterboard, in a unique carbon capture and utilisation process [1]. The hardening process is thought to follow a similar mechanism to gypsum in the manufacture of plasterboard, where the hardening is produced in the recrystallization of gypsum crystals. However, nesquehonite [2], during hardening, converts into hydromagnesite, releasing CO2 and H2O during the transformation. In this work, samples of nesquehonite were hardened by forcing their conversion into hydromagnesite in enclosed cubes [3]. The influence of the curing temperature (50 ℃-80 ℃) and time (0-72 hours) on the compressive strength has been studied and correlated to the conversion of nesquehonite into hydromagnesite [4]. Compressive strength values up to 6MPa are easily achievable in the studied conditions and it was observed that at higher temperatures time has a detrimental effect on the strength. The probable overpressure within the cubes, generated by released CO2 and water at the higher temperatures, is thought to be the main cause for the loss of strength [5].
Keywords: Carbon capture and utilization; Carbon dioxide; Nesquehonite; Hydromagnesite; Plasterboard
Introduction
Mineralisation of carbon dioxide as carbonates is one of the main routes proposed for carbon capture and storage, CCS, with a higher implementation potential, due to the availability of large amounts of minerals and wastes that are suitable for capture. The availability of resources for carbon dioxide storage through mineralisation largely exceeds the emission burden worldwide. However, the excessive cost of treatment, its environmental footprint and the large amount of carbonated wastes generated [6] has made the economy of mineralisation to be dependent on the applicability of generated products through carbonation, which is usually of low grade use, e.g. as construction aggregates or fillers in cement and concrete formulations. This is the case of MgCO33H2O, nesquehonite, NQ. Although it is found naturally [3], it is mainly a by-product of the thermal treatment of Mg-containing rocks such as serpentine [7] and CO2 mineralisation. Its metastable character makes it an undesired intermediate, and other phases, as hydromagnesite or magnesite, are preferred [8] for long-term storage. Nesquehonite is also the main phase formed by interfacing Mg-rich solutions such as reject desalination brine, with dissolved CO2 solution at temperatures around 20 to 40 °C and basic aqueous solutions [1,2,9-11].
A unique application, however, has been recently found, since nesquehonite tends to behave in a very similar way to calcium sulfate phases during plaster manufacturing [12]. In this previous work, this behaviour was studied, and plaster-like products were obtained from nesquehonite. Morrison et al. [11] describes a method in which NQ powder is heated for several hours to remove bound water in a process called ‘thermal activation’. After dehydration, rehydration is performed by mixing a known excess of water to regenerate NQ, producing a paste. The paste is then poured into a mould and develops strength after a few hours. Although the parallelism with the mechanism by which gypsum plasterboard becomes cementitious was used as inception of this development, but the mechanism and phase changes for NQ-based cementitious materials have shown to be of higher complexity [13].
The transformation of NQ into hydromagnesite, HM, seems to be the key step in the development of inner cohesion, probably through a dissolution-reprecipitation mechanism. A second route was therefore developed without thermal activation but just using the appropriate temperature and humidity conditions for the transformation of NQ into HM [12]. This second mechanism is called ‘forced conversion’. This paper focuses in the empirical correlation of NQ conversion and the development of strength, and uses thermodynamic modelling and experimental data to produce a preliminary proposal of the potential hardening mechanism.
Materials and Methods
Nesquehonite preparation
Around six hundred grams of pure NQ were obtained in a 50L batch reactor by mixing 10 litres of MgCl2 (0.5mol L-1) and a mixture of sodium carbonate (0.1125mol L-1) and sodium bicarbonate (0.0125mol L-1). The mixture was stirred for 4 hours at 500rpm at room temperature. A white powder was obtained after vacuum filtration and rinsing and dried at room temperature and atmospheric conditions. The use of sodium bicarbonate helped to reduce the pH, so the co-precipitation of HM could be avoided (as explained by Morrison et al. [11]). All nesquehonite used in the work of this paper was synthesised under the conditions described above. This ensured the comparability of samples.
Hardening of Nesquehonite through ‘forced conversion’
NQ powder is mixed with water, in a water/solid ratio of 0.8, poured into moulds, and the paste is cured at a temperature between 50 and 80 °C for several hours with conservation of water. Hardening occurs in the course of the curing process and, after the fixed time for each sample, samples are demoulded and dried at room conditions for at least a week.
Solids characterization
Dry powder samples were scanned by XRD using a Panalytical X’Pert Powder diffractometer at room temperature ~22 °C (radiation: Cu-Kα1-α2, operating parameters were: 40mA and 45kV, step size: 0.013 ˚2θ, atmosphere: static air). A Hitachi S-520 Scanning Electron Microscope using a 20kV acceleration voltage, to observe the morphology of the samples. The compressive strength of the cubes was measured on a Hounsfield Universal press with a loading speed of 1mm.min-1 and a sensor of 10kN.
Experimental Result
The precipitated solid is XRD-pure nesquehonite, which morphology consists of needles as shown in Figure 1. Once dried, the needles are disaggregated by themselves to a free flowing powder. The dry NQ product is not cementitious per se. In the following sections, the influence of temperature and time in the hardened characteristics of NQ, cured through the forced conversion method, and the development of a pseudo-kinetic model, which correlates compressive strength with temperature and time, will be described. All the hardened products consist of stable mixtures of nesquehonite, dypingite, dypingite-like and hydromagnesite. Table 1 shows the molecular formulas of each involved compound and their density. The only stoichometric difference between dypingite and hydromagnesite is the amount of crystallisation water [14]: four molecules for hydromagnesite, and five for dypingite. Dypingitelike compounds are those with x water molecules in their structure, with 4< x < 5.
figure 1:SEM image of NQ synthesised under the conditions described in “Materials and methods”.

Table 1:Phase composition and density.
Effect of varying temperature on strength development and conversion
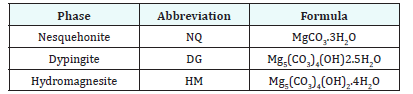
figure 2:Compressive strength, in MPa, of cubes of nesquehonite cured at the specified temperatures and time.
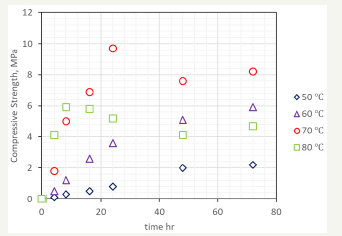
Cubes were prepared with the batch of 600 grams of NQ and cured at a range of temperatures between 50 and 80 °C, for a range of times between 4 and 72 hours, following the experimental procedure described above. The compressive strength valuesof the cubes after curing and drying is shown in Figure 2. As observed, the lower temperatures produce almost a linear dependence between curing time and compressive time, while at 70 °C and 80 °C, the strength is developed rapidly, but longer times do have a detrimental effect on the hardened characteristics of the cube. Crushed cubes were analysed by XRD, and following the mathematical procedure of Morrison [11], the nesquehonite content was estimated through XRD patterns of known mixtures of NQ and HM. The percentage of HM (including also DG and DG-like compounds) at each temperature and time are shown in Figure 3.
figure 3:Estimated content of hydromagnesite of cubes of nesquehonite cured at the specified temperatures and time.
Kinetics of the conversion process
The behaviour of the compressive strength of NQ-based cubes observed in the previous section can be correlated with the amount of HM in cubes [15]. Since the mechanism is thought to involve the redissolution of NQ and further precipitation of HM or DG and DGlike compounds, this would correspond to that observed for plaster, but, instead of recrystallising the initial solid, another more stable phase is obtained. This idea will be further supported through a discussion on the thermochemistry of the process. In the first instance, and due to the behaviour of the curves in Figure 3, a first order pseudo-kinetic model is proposed (equation 1)

Where mNQ is the mass of nesquehonite in the sample, and k is a kinetic constant.
Since this equation would measure a chemical conversion of NQ into HM, the kinetic constant follows an Arrhenious type variation with temperature (equation 2).

Where k0 is a pre-exponential factor and E would correspond to activation energy of the transformation.
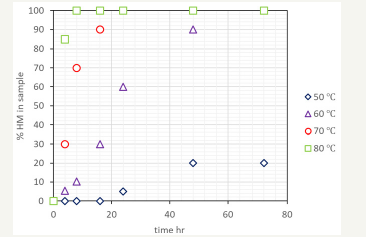
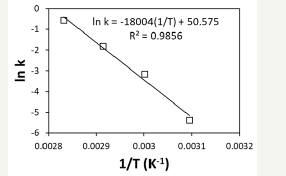
The data in Figure 3 was fitted to the pseudo-kinetic model of equation one. The calculated conversion of nesquehonite shows an accurate fitting to the experimental results, with a correlation factor R2 value of 0.9865 (Figure 4). The Arrhenius plot, in Figure 5, explains the variation of the kinetic value of the constant with a preexponential factor of 9.2·1021 h-1 and an activation energy of 35.8kcal mol-1.
Although a first order kinetics may also correspond to diffusional mechanisms, the Arrhenius type variation of the rate constant indicate a chemical control of the process [16].
figure 4:Experimental and calculated values of the content of hydromagnesite in cubes of nesquehonite cured at the specified temperatures and time.
figure 5:Arrhenius plot of the kinetic constant of the conversion of nesquehonite into hydromagnesite.
In any case, the relation between the curves in Figure 2 and Figure 3 makes possible to correlate the compressive strength with the content of hydromagnesite in a first instance and with temperature and time in a second instance. According to that, we can build a correlation where the detrimental effect of HM content at higher temperatures is taken into account. A differential model is proposed (equation 3):

Where CS is the compressive strength, in MPa, k´1 and k´2 are the pseudo rate constants, in MPa g-1h-1 or MPa g-nh-1, mHM is the mass of hydromagnesite and n is an exponent used to facilitate fitting calculations, which value is set to 0.1.
figure 6:Experimental and calculated values of the compressive strength of cubes of nesquehonite cured at the specified temperatures and time.
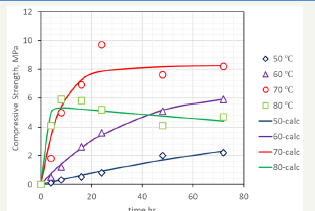
Figure 6 shows the experimental and calculated values with the model of equation 3, which fits the experimental data with a correlation factor R2 of 0.9502 by using equation 1 to integrate equation 3. The kinetic constants where also studied against the temperature and the correlations shown in equations 4 and 5 were obtained.
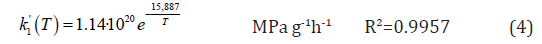
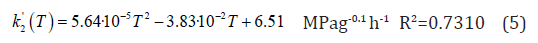
The behaviour observed for k´2 indicates a change of mechanism or the appearance of new chemical process affecting the compressive strength of the sample; the polynomial variation of k´2indicates the change to happen in the range 60-70℃. In relation to this, a study on the chemistry of the process and it relation with temperature, aqueous solution behavior and thermodynamics is presented in a further section below. The correlation built in this work predicts well the behaviour of the studied NQ samples, and, more importantly, correlates the compressive strength with curing temperature and time of NQ cubes in the forced conversion hardening [17].
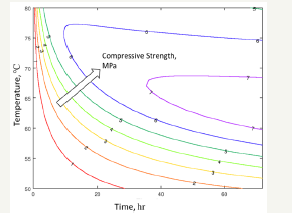
Figure 7 shows a contour plot of compressive strength vs time and temperature. It basically represents what has been observed experimentally: higher temperatures help to the rapid development of strength but have a detrimental effect in the long term. Therefore, there is an optimum temperature and time for strength development. In this way, for instance, the chart could be used to design nesquehonite pieces with at least7MPa of strength by curing samples at 67℃ during, at least, 40hours.
figure 7:Contour plot of predicted compressive strength in the range of 50 ℃ - 80 ℃ curing temperature and 0-72 hours of curing time.
Discussion
Thermochemistry of the conversion of nesquehonite into hydromagnesite
The conversion of nesquehonite to hydromagnesite involves the loss of one molecule of CO2 and 10 of H2O for every molecule of hydromagnesite formed, according to reaction [1]. This loss during conversion is an important driver of structural and mineralogical change and seems to be linked with strength development.
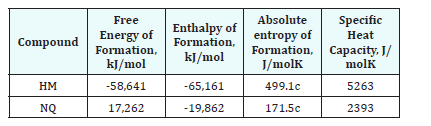
As aforementioned, the curing reaction is based in the transformation of NQ into DG, DG-like or HM. The theoretical influence of the temperature can be studied through the study of the free energy and enthalpy of that reaction. A calculation of thermodynamics was performed using Gibbs Energy Minimization Software (GEMS) [7]. GEMS calculate the equilibrium composition of a mixture of compounds and phases through the minimization of the system Gibbs energy. The calculation of the chemical potential of aqueous species is made using available data in GEMS. For the solids, thermodynamic data is shown in Table 2 was used.
HM thermodynamic data is used as a surrogate for DG and DGlike, since this is a metastable phase and no reliable data is available in the literature. DG or DG-like are thought to be an intermediary phase in the formation of HM, slightly less stable than HM. Therefore, the use of HM as surrogate for DG or DG-like compounds is considered to be appropriate in this qualitative analysis since it represents the final equilibrium state. In all calculations the formation of magnesite, MgCO3, is not considered since there are important kinetic restrictions during its formation, even being the most stable magnesium carbonate form.
The data in Table 2 allows the calculation of the free energy and enthalpy of the curing reaction. This reaction can be expressed in two different ways, depending on the physical state of released water (reactions [2] and [3]):
Table 2:Thermodynamic properties of Nesquehonite and Hydromagnesite GEMS calculations [5,8,13].
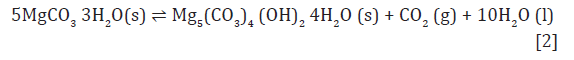
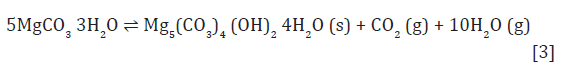
For reaction [2], the theoretical value for the free energy of reaction at 25 °C is -0.4kJ/mol and the enthalpy of reaction is 138.5kJ/mol. For reaction [3], the free energy of reaction at 25 °C is 85.6kJ/mol and enthalpy of reaction is 573.5kJ/mol. The conversion reaction at 25 °C is slightly spontaneous when liquid water is produced, but its endothermic character makes the natural conversion of nesquehonite unlikely at room temperature. However, the conversion reaction is significantly detected at temperatures over 50 °C and the best performance of hardened solids is observed at 70 °C, where conversion of NQ to HM, DG or DG- like takes place in a few hours, meaning that the conversion effectively happens at those temperatures. The theoretical variation of the free energy of formation with temperature can be easily calculated and it is shown in Figure 1. Enthalpies of reaction at temperatures over 25 °C were calculated but are not shown within this document, since they still are positive and vary only around 10% of the value at 25 °C (Figure 8).
figure 8:Free energy of the conversion reaction producing H2O(l) and H2O(g)
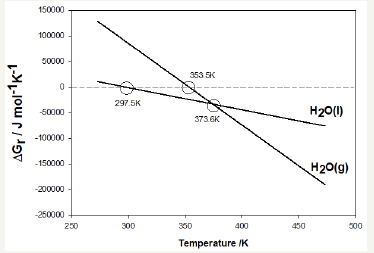
figure 9:Thermodynamic equilibrium composition of NQ cubes with temperature during curing (a) and pressure build-up during conversion reaction at constant volume (b).
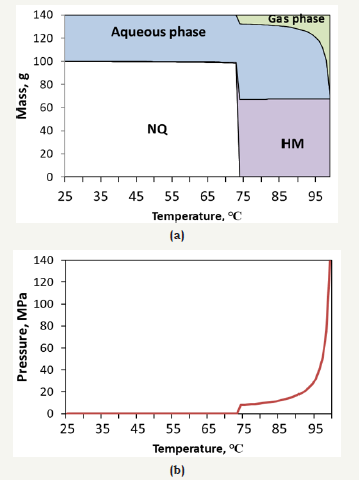
As observed, the free energy of reaction [2], producing liquid H2O, is spontaneous from 297.5K (24.3 °C). Reaction [3] would be the thermodynamically preferred route from 373.6K (100.4°C), although it is favourable from 353.5K (80.3 °C). This temperature coincides with the loss of strength of hardened cubes in the curing process, shown in Figure 2. This may probably be due to the pressure build-up from steam and CO2 within the cubes: reaction [3] produces 11 moles of gas (10 H2O plus one CO2) per mol of HM, DG or DG-like, which may indicate a fast overpressure within hardened samples. Figure 9 shows the theoretical prediction of the composition of a NQ cube (extrapolated to 100 grams of solid). As observed, the assumption of a constant volume would create a massive pressure build-up at temperatures over 80℃, in a first phase due to the release of CO2 and, in a second phase, due to the evaporation of water. A more realistic kinetic transition, as studied in the previous section, would change the transition temperature from the theoretical 75-80 ℃ to 65-70 ℃.
Changes in the solid mineralogy during the curing process are accelerated due to the presence of an aqueous pore solution at temperatures over 50 °C. It is thought that the pore solution composition is key to understand the chemical changes in the solid phase that hardens the solid, probably through a redissolution - recrystallization process, similar as that happening for gypsum hardening [16], and will constitute a matter of further studies on the hardening mechanism.
Final remarks
Samples of magnesium carbonate trihydrate, nesquehonite, were hardened by forcing their conversion into hydromagnesite. It is thought that this conversion built up the strength of the cube samples, and materials composed mainly of hydromagnesite and similar compounds, as dypingite, can easily be manufactured with compressive strengths of around 6 MPa. This is achieved by mixing nesquehonite with water and curing at temperatures above ambient up to 80 °C, such that conversion to hydromagnesite occurs according to reaction [1]. This method, named forced conversion, shows that an increased conversion to hydromagnesite compressive strength increases (Figure 2) and bulk density decreases (not shown). The decrease in bulk density is a result of the loss of material during the conversion reaction, whilst maintaining its shape and cohesion, i.e. keeping volume constant.
Empirically, the mechanism of conversion develops a structure that has cohesion and shows compressive strength. It is known that the strength that gypsum gains after dehydrating and rehydrating is achieved by recrystallization of gypsum; consequently, it is though that the mechanism for nesquehonite cubes is similar, but a different solid phase, hydromagnesite, precipitates. This behaviour would allow also to explain the detrimental effect of fast conversion at high temperatures, since the released CO2 dissolves in the water (added to enhance the conversion reaction or produced during the reaction), and is released as gas at temperatures over 70 ℃, along with steam. The gas pressure builds up within the porous structure of the solid, and probably reduces the strength of the solid if excessive or not properly released at temperatures over 65-70 ℃.
Future work should focus on the further understanding of the hardening mechanism, through a study on the formation of micro and mesoporosity in the solid during conversion, and how this relates to the compressive strength of the samples. Commercial applications would probably require the understanding of the chemistry of the process in order to develop e.g. additives that alter the hardening mechanism, focusing on the design of products that meet conventional standards.
References
- Calera (2012) Sequestering CO2 in the built environment. Slides presentation at MIT.
- Ferrini V, De Vito C, Mignardi S (2009) Synthesis of nesquehonite by reaction of gaseous CO2 with Mg chloride solution: Its potential role in the sequestration of carbon dioxide. J Hazard Mater 168(2-3): 832-837.
- Giester G, Lengauer CL, Rieck B (2000) The crystal structure of nesquehonite, MgCO3·3H2O, from Lavrion, Greece. Mineralogy and Petrology 70(3-4): 153-163.
- Glasser FP, Jauffret G, Morrison J, Galvez Martos JL, Patterson N, et al. (2016) Sequestering CO2 by Mineralization into Useful Nesquehonite- Based Products. Frontiers in Energy Research.
- Hänchen M, Prigiobbe V, Baciocchi R, Mazzotti M (2008) Precipitation in the Mg-carbonate system: Effects of temperature and CO2 pressure. Chemical Engineering Science 63(4): 1012-1028.
- Huijgen WJJ, Comans RNJ, Witkamp GJ (2007) Cost evaluation of CO2 sequestration by aqueous mineral carbonation. Energy Conversion and Management 48(7): 1923-1935.
- Kulik DA, Wagner T, Dmytrieva SV, Kosakowski G, Hingerl FF, et al. (2013) GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Computational Geosciences 17(1): 1-24.
- Langmuir D (1965) Stability of carbonates in the system Mg2-CO2-H2O. The Journal of Geology 73: 730-736.
- Ma J, Yoon RH (2013) Use of reactive species in water for CO2 mineralization. Energy and Fuels 27(8): 4190-4198.
- Mazzoti M, Abanades JC, Allam R, Lackner K, Meunier F, et al. (2005) Mineral carbonation and industrial uses of carbon dioxide. Carbon Dioxide Capture and Storage, p. 319.
- Morrison J (2016) Carbon dioxide sequestration into novel, useful materials: synthesis and properties. University of Aberdeen, UK.
- Morrison J, Jauffret G, Galvez Martos JL, Glasser FP (2016) Magnesiumbased cements for CO2 capture and utilisation. Cement and Concrete Research 85: 183-191
- Robie R, Hemingway B (1972) The heat capacities at low temperatures and entropies at 298.15K of nesquehonite and hydromagnesite. American Mineralogist 57: 1748-1781.
- Sanna A, Dri M, Hall MR, Maroto Valer M (2012) Waste materials for carbon capture and storage by mineralisation (CCSM)- A UK perspective. Applied Energy 99: 545-554.
- Wang X, Maroto Valer MM (2011) Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation. Fuel 90(3): 1229-1237.
- Weiser HB, Moreland FB (1931) The setting of plaster of Paris. J Phys Chem 36(1): 1-30.
- Wilson SA, Dipple GM, Power IM, Thom JM, Anderson RG, et al. (2009) Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada. Economic Geology 104(1): 95-112.
© 2018 Ammar Elhoweris. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






