- Submissions

Full Text
Advances in Complementary & Alternative medicine
Contaminated Waters and Depleted Soils: Impact in Nutrition
Ana Lucia Ramalho Mercé*
Chemistry Department, Federal University of Paraná, Brazil
*Corresponding author:Ana Lucia Ramalho Mercé, Chemistry Department, Federal University of Paraná, Brazil
Submission: December 11, 2023;Published: December 22, 2023

ISSN: 2637-7802 Volume 7 Issue 5
Abstract
Toxic elements can contaminate edible crops either from water (rainfall, wastewater…), air (atmospheric dust, aerial delivery of pesticides, industrial and mine activities, heavy traTic ways, chemtrails…), and soil (contaminated site, industrial and mine activities…). Many transition metals are essential to many enzymatic and proteomic biochemistry, these metal ions being susceptible of being replaced by the toxic ones that bear similar chemical properties. They all end up in human bodies with various nefarious consequences. Metal uptake by roots of the edible crops is dependent on the metal chemistry and its speciation according to redox and pH conditions of the soil, the plants in question and the soil geology. Some chemistry theory concerning acids and bases, very easy to grasp, are presented in order to understand how some metal ions and metalloids can be more tenacious to be detoxified in our body than others, that are excreted easier. These considerations are stated in the Pearson’s Hard and Soft Acids and Bases Theory (HSAB), where soft Lewis acids and bases are relatively large, polarizable atoms, ions, and molecules and hard Lewis acids and bases are relatively small and less polarizable, explaining the synthetic and natural molecules structure acting as antidotes for intoxication chelation treatment and prevention. Some chemical antidotes for heavy metal intoxication are discussed, synthetic and natural ones, the latest present in medicinal herbs and other edible plants.
Keywords:Essential metal ions; Toxic metal ions; Natural and synthetic chelating agents; Synthetic pesticides; Metalloenzymes; Radioactive elements; HSAB theory; Periodic Table; Medicinal herbs
Abbreviations:GSH: Reduced Glutathione; MTs: Metallothionein’s; PhyTs: Phytochelatins; HSAB: Hard and Soft Acids and Bases Theory; DMPS: Dimercaptopropane Sulfonate; DTPA: Diethylenetriamine Pentaacetic Acid; Metal ions: Po: polonium; Pu: plutonium; U: uranium; Cr: chromium; Tl: Thallium; Na: Sodium; K: Potassium; Mg: Magnesium; Ca: Calcium; Cr: Chromium; Mn: Manganese; Fe: Iron; Co: Cobalt; Cu: Copper; Zn: Zinc; Mo: Molybdenum; C: Carbon; H: Hydrogen; N: Nitrogen; O: Oxygen; P: Phosphorus; S: Sulfur; Cl: Chloride; Mn-SOD: Manganese Superoxide Dismutase; GPx: Glutathione Peroxidase; ThxRed: Thioredoxin Reductase; RDI: Recommended Daily Intake; ROS: Reactive Oxygen Systems; POEA: Polyoxymethylene Amines or Polyethoxylated Tallowamine; DDT: Dichloro-diphenyl-trichloroethane; GABA: γ-Aminobutyric Acid; Ca-ATPase: Calcium -ATPase; Mg-ATPase: Magnesium-ATPase; DMPS: Dimercaptopropane sulfonate; DTPA: Diethylenetriamine Pentaacetic Acid; DFO: Deferoxamine; Deferasirox: (4-[3,5-Bis(2- Hydroxyphenyl)-1,2,4-Triazol-1-yl]-Benzoic acid); ppm: Parts Per Million, milligram/Liter (mg/L); ppb: Parts Per Billion, microgram/Liter (μg/L); POEA: polyoxyethylenamines (Polyethoxylated Tallowamine); ROS: Reactive Oxygen Species; OS: Oxidative Stress; DDT: Dichloro-Diphenyl-Trichloroethane; POPs: Persistent Organic Pollutants; miR-155: Micro RNA 155 ; cyt b561: Cytochrome b561.
Introduction and Brief History
Metal ions are commonly found described in the literature as part of a need in nutrition. Knowledge of their roles is the most important given their presence in metalloproteins, metalloenzymes and in the human biochemical detox process, to name a few. One must bear in mind, however, that every substance can be deleterious or even deadly if consumed in an inadequate - more in a higher than in a lower-concentration [1]. This review aims to Till in this gap and also bring to the Nutrition professionals and laymen public an insight of how metal ions can have a very toxic or a very healthy overall impact in our biological systems. In the well-known statement - …”depending on the concentration everything can be toxic”- it is important to remind that there are inorganic and organic substances that are highly toxic in any concentration, even mere traces. As the world population is searching for more and more cutting-edge technology without asking how and with what material they are being made of, it is a matter of urgence that this subject is further studied and discussed to complement this first review.
Foods are more and more depleted of their nutrient elements as the intensive and monoculture agricultural methods are ubiquitously found in the “modern” way of producing foods. Also the nowadays inflicted twisted values considering the more industrialized the food, the safer it will be, has brought a tsunami of undernutrition and obesity in the population. It is important to distinguish between “being nourished from being fed”. Nature, in her great inteligence can, sometimes, chemically replace one metal ion that is lacking in the soil by another that would perform the same biochemical role. But no living being in this planet is ever prepared for the deluge of toxic wastes we have been damping on the planet, turning available the most toxic elements to our external and internal environment. The enzyme systems present in Amanita muscaria as well as in nitrogenases (from Azotobacter) and Bromo peroxidase from marine algae are dependent on vanadium. One species of Azotobacter (Azotobacter chrooccocum) has two nitrogenase systems - one vanadium dependent and another one, molybdenum dependent [2]. Amanita muscaria, although not edible for humans serves us as an example of this capacity of using other metal ion when the needed one is not available. This mushroom needs vanadium to synthesize the amavadin - a complex of oxovanadium (IV) and two molecules of N-(1-carboxyethyl)-Nhydroxyalanine [3]. In the absence of vanadium in the soil, it uses molybdenum. Another example of use of these 2 metal ions is in the Methanosarcina barkeri strains, anaerobic methanogens, for nitrogen Tixation [4]. Essentiality of trace element micronutrition are not only very important to the human homeostasis but increasingly important in a pregnant woman. Trace elements are crucial to avoid complications in pregnancy as “induced hypertension and preeclampsia, preterm delivery, anemia and smaller fetus compared to the gestational month of pregnancy”. Micronutrients supplementation is a must in pregnant women by supporting the metabolic demand of the growing fetus [5]. The interchange of metal ions in nature will always follow in order to achieve the same biochemical properties of the desired Tinal product. In the misfortune of absence or low concentration of the desired metal ion available, it will happen the uptake of one having similar physico-chemical properties regardless of being toxic to life. That discussion will follow.
Discussion
Nutrition and the periodic table of the elements
In Figure 1 a simple sketch of the Periodic Table of the elements is displayed showing how the elements were classified according to their main properties considering the atomic number, as well as the atoms’ electrons. In general, the more the elements are placed on the left side, the more they behave as metals. On the other hand, the more placed to the right-side region of the periodic table, the less the metal character. This rule applies better for the first 2 rows in the Table where the last Tilled orbitals are the s and p and early d. In the central portion of the Table lie the transition metals. Many of them, due to their particular chemical behaviour and electron configuration, have dual behaviour. They are the most important for the more specialized life biochemistry. On the other hand, some of them are the most dangerous for this same life, either in the air, water or soil. These three environments, herein considered separated for academic purposes, have 100% impact in the quality of generated food and other edible resources, and consequently in the nutritious or toxic - sometimes lethal - values in them.
Figure 1:Periodic table of the chemical elements. The University of Nottingham has a website for periodic videos of the elements which can shed light in the main properties of them.
Essential elements
In the periodic table, Figure 1, at least 20 elements are essential to the biochemistry of life - metals {sodium (Na), potassium (K), magnesium (Mg), calcium (Ca), chromium (Cr) [6], manganese (Mn), iron (Fe), cobalt (Co), copper (Cu), zinc (Zn) and molybdenum (Mo)}; and non-metals {carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P), sulfur (S), chloride (Cl-)}….The Biochemistry of metal ions are basically related to their complexing capacity (binding / chelating ability), speciation and mobility. The more they are able to form strong bonds with ligands, the less they are mobile. The more mobile they are, the less prone they are to bind strongly to potential ligands. For us to have a general idea, the Irving-Williams series states the differences among the divalent Tirst row of transition metal cations and the order of stability of their metal complexes with organic ligands - Mn(II)< Fe(II) < Co (II) < Ni(II) < Cu(II) > Zn(II) [7]. The more stable redox numbers they have, the more different species they can present according to either pH or redox conditions. Glutathione (reduced-GSH) and Metallothionein (MT=intracellular metal binding proteins) levels have major roles in human and also Phytochelatins (PhyTs) in plant Biochemistries. These MTs and PhyTs, among other very important roles in the Biochemistry of life, are the sole molecules that can bind toxic metal ions, being the main part of the detox system in both plants and animals, without any harm to the living system. For example, non-MTs-bound cadmium is toxic and causes toxic harm to the cells. In plant and soil biochemistry divalent metal ions are critical catalytic cofactors, as all living systems depend on them for their enzymatic action on phosphoryl transfer reactions [8]. So, the need for a divalent metal ion for these reactions to take place, if the soil or water around the plant are contaminated with toxic metal ions having similar chemical behaviour, the edible plant will absorb them, turning into a silent toxic food or medicament in the case of medicinal plants.
Toxic and Essential Metal Ions in Nutrition, Soil and Waters
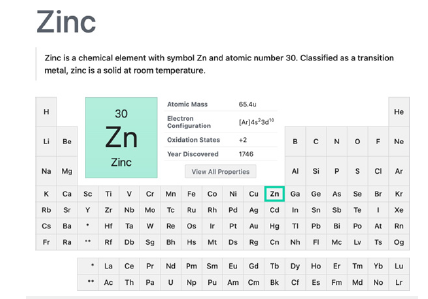
Zinc
Another example (Figure 2), zinc presents structural cofactor, catalytic action in a few hundred enzymes and regulatory cellular bio-functions in human biochemistry (zinc is essential to all forms of life). The roles of zinc ion in cellular regulation are unique among the essential transition metal ions. Examples are their essentiality in bone formation, amino acids, cholesterol and carbohydrate metabolism. Among approximately 3000 zinc proteins encoded by the human genome, there are hundreds in all classes of enzymes (oxyreductases, transferases, hydrolases, lyases, isomerases and ligases), and hundreds of zinc Tinger proteins. This kind of zinc protein has functions that are extraordinarily diverse and include DNA recognition, RNA packaging, transcriptional activation, regulation of apoptosis, protein folding and assembly, and lipid binding [1,9-13] as a free metal ion after digestion, zinc binds to endogenously secreted ligands, forming coordination complexes before their transport into the enterocytes in the duodenum and jejunum. About 70% of the zinc in circulation is bound to albumin, and any condition that alters serum albumin concentration can have a secondary effect on serum zinc levels.
Figure 2:Zinc transition metal element summary in the periodic table. Courtesy of: National Center for Biotechnology Information. “PubChem element summary for Atomic Number 30, Zinc” PubChem.
Hepatic and intestinal Metallothionein synthesis-the main Zn buffers in mammalian cells - is stimulated by dietary zinc supplementation as MT is a metal storage protein that is present in serum at a low concentration, and its circulating concentration appears to correlate with zinc intake. Phytic acid, although considered a natural antioxidant, forms insoluble complexes not only with minerals but also with proteins, enzymes… The absorption of iron, zinc, calcium, and magnesium, especially in people with higher nutritional demands and with not enough intake, or mineral and trace elements deficiencies, are hindered by phytic acid. This phytic acid also binds strongly mainly zinc in the gastrointestinal tract. Whether zinc elicits pro antioxidant, anti-inflammatory, or antiapoptotic effects depends on its concentration [1,10-13].
Nearly 10% of all plant proteins belong to the zinc proteome. Being a d10 Tilled electron transition metal ion 2+, it is not redox active and thus, not prone to form free radicals, so it can be safely used in enzymes or other proteins interacting with DNA or RNA; the inteligence of life. According to the HSAB theory (refer to the section on HSAB-4.1.), zinc is a borderline acid having either strong affinities for soft bases, such as sulfur ligands, or for hard bases, such as oxygen and nitrogen ligand sites, all present in proteins; very often presenting a tetrahedral coordination, with cysteines and histidine’s being the most common zinc ligands: zinc Tinger proteins contain two cysteine and two histidine residues. In other DNA-binding proteins, two Zn atoms are coordinated by six cysteines. Also in plants, mitochondrial Zn proteins include ribosomal proteins, tRNA ligases, and DNA polymerases [14].
Among the essential nutrients in plants, zinc is one of the most important, along with manganese and iron. Zinc is used in plant biochemistry in the roots (zinc storage and transfer to aerial parts), in the exchange of xylem and phloem, in fruits and in the RNA and protein synthesis in general. The essentiality is linked not only to being a cofactor in enzymes, but also in auxin (a group of plant hormones that regulate growth) formation, membrane stability and participation in the biosynthesis of chlorophyll [8,15]. According to the studies reported in reference [8] the excess of zinc, causing a toxic effect in the plant in general, can produce a list of undesired effects, from oxidative stress, growth inhibition and decrease in seed germination, to chlorophyll degradation and disruption of enzyme activities. In soil, there is competition among the many soluble ions and the soil factors affecting zinc Phyto availability (for the majority of mineral nutrients as well) which are pH, redox value, microbial activity, cations and soil organic matter. Relative proportion of zinc depends on soil texture, pH, soil zinc level, and the presence of competing cations such as iron and magnesium. Zinc is preferably stored in plant roots due to the presence of metal chelator proteins - Phytochelatins, nicotiana mine, metallothionein’s… [15].
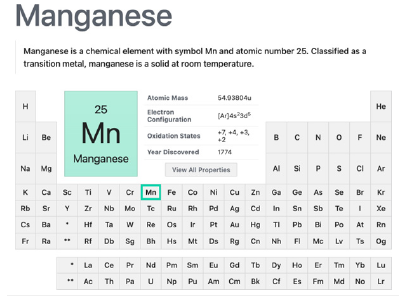
Manganese
Considered as one of the essential metal ions to life, manganese can present many redox states (eleven - from -3 to +7), being the 2+ the most important in biochemistry, participates in electron transfer reactions along with the activation, storage and transport of molecular oxygen, and other important biological processes. Also, it can exchange with zinc(II), iron(II) and cobalt(II) in the coordination sites of enzymes keeping their biological functions. Another main role of manganese as an essential metal to life is acting as an enzyme cofactor or as a metal with catalytic activity in biological clusters. Manganese redox states +4 and +3 in soils are not water-soluble species due to forming insoluble oxides, paving way for manganese (II) to perform the biochemical roles and also to establish the equilibrium and availability of soluble - insoluble manganese species (Figure 3). Manganese deficiency in animals produces several adverse effects, skeletal abnormalities and growth alterations, ataxia, among others. In humans, a review [16] presents studied manganese in the bone health in humans. It was found that manganese is an important orthotropic element (a nutrient for osseous tissues), not only in stimulating the bone matrix synthesis but also in the calcification in general. Human manganese absorption in food has a very little efficiency (less than 4%), happening in the small intestine [16-19].
Figure 3:Manganese transition metal element summary in the periodic table. Courtesy of : National Center for Biotechnology Information. “PubChem Element Summary for Atomic Number 25, Manganese” PubChem.
Like any other substance, manganese can also be toxic at high doses or exposure to high concentration via inhalation or oral contamination. Under excessive manganese concentration the central nervous system produces adverse neurological effects, called manganese [20]. Manganese is essential for photosynthesis, playing the major role in chloroplast function. It is also an active participant in enzymes, as a d-block metal. Manganese Superoxide Dismutase (Mn-SOD) is an enzyme that promotes the dismutation of superoxide radical anions. In plants this Mn-SOD requires only manganese and is an important cofactor in enzymes involved in the isoprenoid biosynthesis (terpenes formation), the one producing the secondary metabolites for plant protection and communication [18]. Manganese is an essential element found in rocks, soil and water. Dietary sources of manganese including wheat, brown rice, spinach, pineapple and soybean, being food intake the main source of it, the Recommended Daily Intake (RDI) is 5mg/day - even for pregnant women [5].
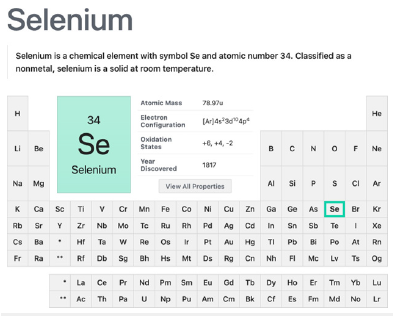
Selenium
Selenium is critically essential (Figure 4) due to its presence in various sialoproteins and selenoenzymes - among which the very important detox and antioxidant Glutathione Peroxided (GPx), the thioredoxin reductase (ThxRed), sialoprotein-P and iodothyronine deiodinases. Selenium also provides anti-inflammatory action and is an active factor in the thyroid hormone synthesis and is also related to the cardiovascular systems. The RDI for this nonmetal is 60μg/day, and 70μg/day for pregnant and during lactation women. It is very important to know if the soils in which the sources of selenium foods grow (Brazil nuts, cereals, eggs, fruits and vegetables), have selenium on them, otherwise, those selenium containing reputed foods will be blank in this element [5,21]. During pregnancy, oxidative stress increases hence the women bodies rely on antioxidants. GPx and ThxRed, selenium antioxidant enzymes where selenium is bonded to cysteine, will considerably increase with selenium supplementation, reducing placental oxidative stress (reactive oxygen systems-ROS) and therefore, pre-eclamptic episodes [5,21].
Figure 4:Selenium nonmetal element summary in the Periodic Table. Courtesy of: National Center for Biotechnology Information. “PubChem Element Summary for Atomic Number 34, Selenium” PubChem.
Toxic Substances: Heavy Metal Ions, Metalloids and Pesticides
Hard and soft acids and bases
In order to state better the affinities of metals towards ligands, the concept of Hard and Soft Acids and Bases will be discussed (HSAB). Many research works have been published on heavy metal ions. The majority of them have a great impact if not causing death in so many biochemical and physiological processes, that it would be reckless not to consider them in our daily lives in food or in medicinal plants. They can have devastating effects on living beings or being responsible for damages ranging from permeability changes in the cellular membrane, oxidative damages in cells either in the form of lipid peroxidation and oxidative DNA damage, being highly tumorigenic potential, inducing chromosomal and apoptotic and neurogenic chromatin changes and having genotoxic effects [22]. Notwithstanding all the industrial and army production, main sources of heavy metals waste nowadays, the plastic shed production systems of human edible vegetables are another source of contamination for the soil under these plastic cover greenhouses.
All heavy metal ions have a high mobility in the environment and, depending on the cultivation and management, pH, redox potential, salt and nutrient accumulation, and less organic matter in the soil can alter and enhance the migration of these metal ions in soils under those plastic sheds [23]. Not only all these described industrial activities can make metals available for readily contaminate soils, but also pesticides - in general reinforcing the toxicology of heavy metals [24-26]. Originally formulated with heavy metals, can contribute to ubiquitous contamination in soil and water. These chemicals - insecticides, fungicides, bactericides, herbicides or rodenticides-are mostly designed to impair physiological activities of target organisms. Bear in mind that many of these physiological activities have similar biochemical routes and the same nefarious action on us humans [27]. Human activities, by far the most polluting sources of heavy metals, together with natural volcanic events have brought to surface these substances by contributions according to Figure 5. For more details of heavy metals together with pesticides effects in human health see figures in [24].
Figure 5:Some sources of human exposure to xenobiotics (Adapted from reference [24]).
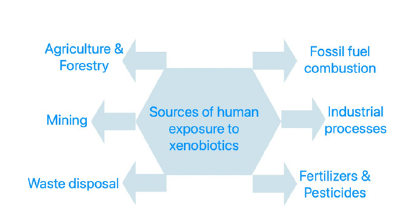
Plants can uptake and liberate heavy metal ions among the essential ones they need in order to perform all their biochemical reactions for survival. Metal uptake from the soil solution depends on availability and the metal ions being in the proper speciation form, according to the soil pH and composition. By the same specific and well-organized mechanisms the vegetable root, through chelating agents, pH and redox reactions, can uptake micronutrients in order to survive, at very low concentrations. The same can happen to toxic elements, that can be biodegraded, or bio transformed into inert forms in their tissues. All these elements will eventually return to soil or end up contaminating those who fed on the still contaminated vegetables [28]. The same accumulation effect of heavy metals with also arsenic was studied in Tish, being in some studies “routinely found” even in the “remote” Phase river in the Tibet Plateau [29,30]. Biochemical researchers rightly suspected long ago of the possible synergistic enhanced toxicity approach of both heavy metals and pesticides in the human body. Many biological effects of some of them are listed in the reference [30].
Glyphosate is well known as a pesticide agent against herbs killing all herbs except the transgenic edible crops developed by many biochemical agrobusiness giant companies. Although much concern with glyphosate-based pesticides debate was the top subject of the decade, it was found that not only glyphosate cannot reach the core of the plant to perform the desiccation, for instance, of leaves, if other major components are not present, but also the toxic effect of these herbicides are not due to glyphosate alone. Having not been found to be as toxic as its reputation, glyphosate is only capable of living up to its job when other compounds, for instance POEA (polyoxyethylenamines (polyethoxylated tallowamine)), are present. This late class of compounds are petroleum derived surfactants, promoting the entering of the herbicide into the vegetal cells. Nonetheless being conceived and used as such, they were found to be more toxic than the glyphosate-derived compounds themselves [31,32].
In the case of a miracle event, it would suffice for these agrobusiness giants to “announce” they would change the glyphosate-derived ingredients for another one in pesticides for the public’s assurance, when in fact the biggest toxic problems reside in the synergistic effect between glyphosate and the other formulation compounds [32]. It is not enough to emphasize the need to consume only organic foods. Not only did the scientific literature show the high human toxicity of pesticides, but also showed many heavy metals and metalloid present in their formulation, like arsenium, cobalt, nickel and lead. (refer to Figure 6 in [31]). Pesticide poisoning accounts for many worldwide deaths. Examples are organophosphate as well as carbamate compounds used as pesticides which inhibit acetylcholinesterase, that is, they attach to the serine hydroxyl group of acetylcholinesterase preventing acetylcholine to be broken down. Acetylcholine being prevented to be broken down and recycled, leads to excessive levels of this neurotransmitter at the skeletal neuromuscular junction and those synapses where acetylcholine receptors are located [33,34].
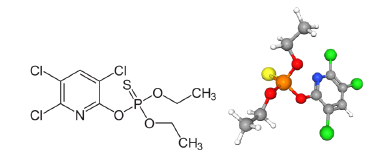
Figure 6:Planar and 3D representation of the pesticide chlorpyrifos chemical struture. IUPAC name: O,O-Diethyl O-(3,5,6-trichloropyridin-2-yl) phosphorothioate. (Courtesy of: NEUROtiker–Own work, Public Domain, and the 3Drepresentation, of PubChem. C=gray ball; H=white ball; S=yellow ball; O=red ball; Cl=green ball; P=orange ball; N=blue ball, and sticks are bonds among the elements; two sticks in the same direction, represent a double bond between two atoms.
Organochloride pesticides (since the synthetic DDT=Dichlorodiphenyl- trichloroethane was launched in the world - in the 1940s) are present and can cause main effects on the central nervous system. Ingestion of paraquat, for instance, was reported to cause corrosive injury to the gastrointestinal tract, renal tubular necrosis and counting. Some of them are GABA antagonists while others are inhibitors of calcium ion influx and/or Ca-ATPase and Mg-ATPase causing release of neurotransmitters [27,33]. Refer to Table 3 in reference [27] for more details. Aluminum phosphide, a highly toxic pesticide, has a mortality rate ranging from 37% to 100%, causing inhibition of mitochondrial cytochrome C oxidase leading to pulmonary and cardiac toxicity [27,33]. This aluminum phosphide, in contact with the air moisture, liberates phosphine (IUPAC: phosphene), a highly toxic colorless pyrophoric respiratory gas . Ethylene dibromide is equally toxic - producing liver and renal toxicity and being almost uniformly fatal [33].
This is to speak only about a few but surely all of them are endocrine disruptors by the same nature they were created for. The biological nefarious effects of both pesticides and any toxic atoms in the periodic table, either heavy metal ions, metalloid or radioactive compounds, are not completely determined since each day, a different combination of toxic cocktails is put out by the agrobusiness industry. A very interesting mini review is worth mention, among others, published in 2017, stating some “synergistic effects of heavy metals and pesticides in living systems”. (refer to [34]) Some of these toxic synergistic effects of both metal ions and pesticides can be summarized as the following. The d orbital partially Tilled elements in the periodic elements table-in Figure 1, transition elements or transition metals - encompasses the most important / essential metals in biology but also some of the most toxic. They are cations that form stable complexes with a myriad of donor of electrons because of this partially Tilled electron orbitals. Others like zinc, cadmium and mercury are d-Tilled metal elements (d10), but also fall in the category of either essential (the Tirst) and toxic ones - cadmium and mercury [34]. The route of exposure of both heavy metals and pesticides are very similar, creating synergistic effects in the case of contamination of these types of toxics. The least of the damages are cell oxidative stress and enzyme inhibition, being the worst-case scenario, DNA damage - impaired DNA replication, cell death and genotoxicity, impaired transcription during gene expression and abnormality in the obtained proteins, among others possible [34].
For example, the exposure of chlorpyrifos (organophosphate trichloro pyridinyl pesticide - patented in 1966), and cadmium decreases the mitochondrial potential and increases the production of ROS leading to oxidative stress [34]. Chlorpyrifos, (or Lorsban - IUPAC name: diethoxy-sulphapyridine-(3,5,6-trichloropyridin-2- yl)oxy-λ5-phosphane), is of acute toxicity class 3 for living tissues, acting on cholinesterase inhibition (increasing the amount of acetylcholine present in the body will trigger symptoms of overstimulation of the parasympathetic nervous system, e.g. bradycardia, miosis, diarrhea…), and cyanosis and blurred vision, is a combustible substance. This pesticide is toxic to birds and other wildlife and extremely toxic to Tish and aquatic organisms [35,36]. Carbon to chloride bonds in the molecule of chlorpyrifos, for instance, (Figure 5) are very strong and resistant to degradation. This kind of pesticides is classified as Persistent Organic Pollutants (POPs), being bio-accumulative, bio-Magnitude and with a likelihood of long-distance displacement in the environment. This persistence is due to them being hydrophobic and as such, accumulates in the environment (in sediments, soils and plants) as well as in the fatty living tissues [37].
A 2016 review article [38] studied the impact in medicinal plants - bioactive phytochemical containing herbs - of both toxic heavy metals and pesticides, presenting tables with valuable data. Another similar study [39] showed that against all odds, wild medicinal herbs were found to have higher contaminant levels of both toxic metals and pesticides than the cultivated ones, collected all over China. Up to forty-two different pesticides were detected in 36% of the samples and all 4 heavy metals (cadmium, chromium, mercury and lead) and 1 metalloid (arsenic) studied, in 34% of the samples. The synergistic worse toxic effect of both pesticides and toxic metal ions was also studied in a main drinking water source in China. In this article, six metals and a metalloid above legal values (copper, zinc, cadmium, nickel and arsenic) and two pesticides (atrazine - organochloride and an endocrines disruptor - and acetochlor - chloroacetanilide derived pesticide) were detected posing environmental and severe health issues risk to humans [40].
According to the conclusion in reference [27], in order to have a safer environment and food the use of alternatives to pesticides, nonetheless involving more manual labor work like “manual removing of weeds, covering them with plastics, removing pest breeding sites…cropping native species that are naturally more resistant to native pests and supporting bio-control agents such as birds and other pest predators. Consumer awareness should be brought up among people in concern with the long-term harm caused by pesticides”. These practices are all encompassed in dynamic and organic / biologic edible plants cultures. Although lead, mercury, aluminum and cadmium are the most notorious toxic metal ions in use today and the most studied, there is an array of others [41,42]. Among them thallium, uranium, chromium (especially Cr(VI)), polonium and plutonium, plus all manmade elements (or synthetic elements, 26 out of 118 elements up till now in the periodic table, produced by nuclear fusion), are neither commonly heard nor studied but, nonetheless, have lethal effects and unsuspectedly, are ubiquitous around us. Many elements in the periodic table, even at very low concentration (ppm or ppb) can wreak havoc in the living cells of animals. Some among the most toxic, can enter in food chain and how they start to damage the body and which natural and synthetic substances can help release and excrete them, are discussed below.
Thallium
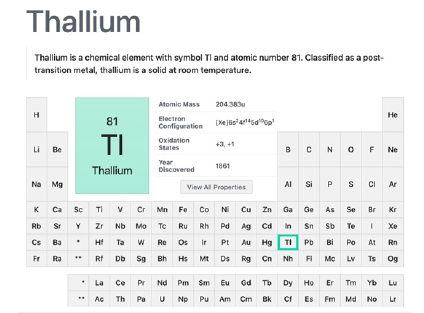
Discovered by William Crookes in 1861, thallium (Figure 7) is employed in the electronic industry in the production of electronic devices, Tiber optics, camera lenses, switches and closures. Combined to mercury (8% thallium) decreases the melting point by 20 oC lower than mercury. alone, providing use for low temperature thermometers and switches. It is largely used as a catalyst for organic synthesis, and it was during my master’s in science in Organic Chemistry development that I knew this very toxic metal ion was indeed commonly used in our daily life [43]. Believe it or not, thallium acetate was once recommended by the medical profession to treat ringworm of the scalp, to suppress “night sweats” in tuberculosis cases, was prescribed orally to remove body hair (cosmetic explant) and as a pesticide [44]. The ill will of authorities in the permissive use of thallium in the early 1950’s and 1960’s even with the public knowledge of its toxicity, can be attested in a canny detective thriller novel - The Pale Horse, published in 1961 by Agatha Mary Clarissa Mallow an, alias Agatha Christie [45] - where thallium was used in the plot as the death weapon.
Figure 7:Thallium post-transition metal element summary in the periodic table. Courtesy of : National Center for Biotechnology Information. “PubChem Element Summary for Atomic Number 81, Thallium” PubChem.
Thallium presents 2 possible oxidation states, 1 and 3, being 1+ the more stable in aqueous solutions [46]. As an example of how little was researched on thulium’s toxicology, in reference [47], the authors say : ”Thallium is considered a cumulative poison that can cause adverse health effects and degenerative changes in many organs. The effects are the most severe in the nervous system passing through causing alopecia as an atypical feature of thallium toxicity. The exact mechanism of thallium toxicity still remains unknown, although impaired glutathione metabolism, oxidative stress, and disruption of potassium-regulated homeostasis may play a role. One thing about thallium can surely be said, it is one of the most if not the most toxic among heavy metals - more toxic than lead, mercury, cadmium or arsenic. The lack of data about mutagenic, carcinogenic, or teratogenic effects of thallium compounds in humans calls for further research.” [47] And from 2010 up to now (2023) not much has changed [48]. The ionic radius of thallium is very close to the potassium ion, and this similarity was shown to pose biological affinity between them [44,49].
This similarity makes thallium capable not only to directly enter cells, as it is readily absorbed by the skin, but also to substitute K+ in the activation of Na+/K+ ATPase, interfering in this enzyme proper energy production resulting in the swelling of the mitochondria and Finally leading to cell’s death. In other K+ metabolic activities like in the activation of pyruvate kinase and aldehyde dehydrogenase enzymes, or in the stabilization of ribosomes, among others, thallium has been shown to replace K+ with dire biological consequences [43,44,50-52]. In pregnancy, the least one can expect if the mother is contaminated with thallium is gestational diabetes mellitus [52]. Thallium poisoning also presents a wide variety of toxic manifestations: Hair loss, gastrointestinal, central nervous, cardiovascular and renal systems as well as in the skin [33]. Certain level of contamination pesticide multi-toxic infected symptoms in living beings are not always known by medical practitioners, as many are not trained to recognize them as such, making the diagnostic underestimated and not properly treated for metal or pesticide exposure.
Uranium
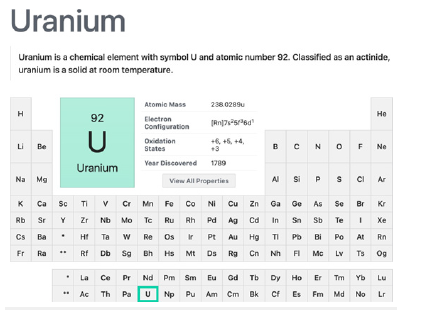
Discovered in 1789 by Martin Heinrich Klaproth, this is a natural element (Figure 8). Due to need to concentrate one of its isotope for being used as a military weapon or fuel in a nuclear reactor, the industrial process involved, the waste dumped in the planet and the residues in atomic bomb explosions and leaks in nuclear reactors, made this toxic radioactive element more concentrated in the surface of the Earth than wanted [46,53-55]. Its presence in the air and in the soil, especially the so-called depleted uranium (depleted of its isotope 238) is reported and is the main source of contamination of crops and humans [56-59]. In 1985, the NIH published in the site the concentrations of uranium found in some foods in the USA. The beef liver, beef kidney, table salt and parsley were the most contaminated at that time [59]. More recently, the main source of uranium contamination is water as a needing drinking resource. Recent research work showed that the mine activities in some specific areas in the USA have contaminated the drinking water resources. The work also shows the different criteria among countries on the tolerated maximum amount of uranium in the drinking water, putting the USA among the highly tolerant countries towards this radioactive mostly toxic pollutant [60].
Figure 8:Uranium actinide metal element summary in the periodic table. Courtesy of : National Center for Biotechnology Information. “PubChem Element Summary for Atomic Number 92, Uranium” PubChem.
Chromium
Nicholas Louis Vauquelin discovered chromium in 1798 (Figure 9). Presenting the oxidation states 6, 3 and 2, Cr is largely mainly used, producing a very toxic waste effluent, in the tanning industry, in the production of stainless steel, pesticide, dyes, aircraft fuselage contributing to contamination not only of water, but soil as well [6]. Chromium III is an essential trace element for insulin regulation and glucose metabolism, but chromium VI is toxic in any uptake concentration. There was a time (work published in 2013 [61]), very unfortunately, when due to scarcity of water in certain regions untreated domestic and industrial effluents were used to irrigate crops, obviously led to accumulation of heavy metals in soils, chromium included [62].
Figure 9:Chromium transition metal element summary in the Periodic Table. Courtesy of: National Center for Biotechnology Information. “PubChem Element Summary for Atomic Number 24, Chromium” PubChem.
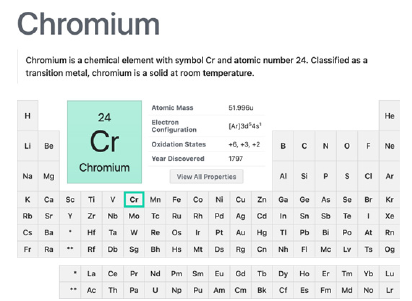
The trivalent oxidation state (Cr(III)) is also present in foods as the more bioavailable Cr(VI) can undergo any reduction process, so studies involving determination and quantification of Cr in foods or in the environment, must take into account the speciation on site. Due to different positive hydroxide compounds Cr(III) forming stable complexes with alumina-silicate clays and laterite soils and with fulvic and humic acids, the mobility and bioavailability of this ion is very impaired. On the other hand, Cr(VI) forms more abundantly negative compounds leading to not being cheated by either clay or humic acid soils (both with negative charge nature) so, as pH rises, the bioavailability of Cr(VI) is increased [63]. The biochemistry of plants seems not to use chromium in any form, but it can uptake them from water or soil [6,64,65].
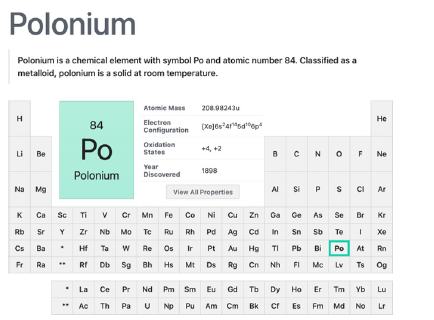
Polonium
Discovered in 1898 by Marie Curie and named after her native country, polonium is an alpha emitter radioactive element therefore being highly toxic to human beings (Figure 10). As a single gram of this element can reach 500 oC as a result of this alpha emitting ability, it is a useful resource of heat for space equipment.
According to the Royal Society of Chemistry [66], as it is a very rare element, it is obtained by bombardment of bismuth and the decaying of one of its isotope to form polonium. All produced polonium in the world comes from Russia. Polonium presents three possible oxidation states (6,4 and 2) and two isotopes 209Po and 210Po. Even since 1964, polonium (210Po) has been reported in the literature as a source, coming either from air or water, of contamination of crops, being the plant tobacco the one studied in this research [67]. Many other spotted polluted sites with polonium can be found near phosphate fertilizer industries, like these reported in Poland [68,69]. As phosphate fertilizer contains higher amounts of uranium than natural soils, these radioactive metals can reach our food chain through grains, vegetable and meat [70]. This poisonous metal ion isotope has come to the public attention when a series of contamination in the center of London was discovered after the assassination by polonium poisoning of a former Russian intelligence officer, Alexandre Litvinenko in 2006 [71].
Figure 10:Polonium metalloid element summary in the periodic table. Courtesy of: National Center for Biotechnology Information. “PubChem Element Summary for Atomic Number 84, Polonium” PubChem.
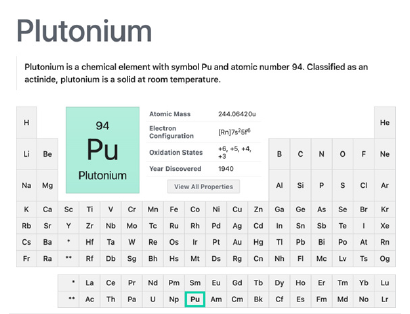
Plutonium
This metal ions were discovered by Glen Seaborg and colleagues in 1940 (Figure 11). It has 3 isotopes (238Pu, 239Pu, 240Pu, 241Pu) and 4 oxidation states, 6, 5, 4 and 3. Used in several of the Tirst atomic bombs, is still employed in nuclear weapons and is a key element in the development of nuclear power. The way it can reach soil and crops is ubiquitous, having been studied in many works, among them one where the researchers found that plutonium was banded mostly by organic matter (67% Pu-uncultivated and 36%-arable soils), but nonetheless “the amounts of plutonium combined with the labile fractions (ion-exchange and carbonate) were approximately 4 times higher for the arable soils than for uncultivated ones… “ [72]. In northeast China, the way the levels of 239,240Pu are gathering is through, among others, global fallout of atmospheric nuclear weapons tests, topography and precipitation indexes, monsoons [73,74].
Figure 11:Plutonium actinide element summary. Courtesy of: National Center for Biotechnology Information. “PubChem Element Summary for Atomic Number 94, Plutonium” PubChem.
The way toxic metal ions can enter the food chain may occur in contaminated soils used to grow food are the water, the plant systems biochemically and physiologically speaking, and other geological conditions playing a major role in this contamination. Taking the biochemistry and physiology of plants as an example of uptake of plutonium from plants, one observes that it can be through the uptake way for iron, as these two metals have similar chemical properties. An experiment in hydroponically grown maize showed definitely interactions between an essential metal (iron) and this toxic one, plutonium [75]. The end of all wasted industrial materials are invariably, the air, water or soil, or in the worst-case scenario, all of them. Our nutrition sources are from all of them, as the basic nutrition for humans and other animals comes from plants, which in turn, feed from water, air (oxygen), minerals (metal ions included) and macronutrients from the soil. The cyclic recycling of nature will inevitably put some of these toxic metals in our table and we need to know about how to deal with them [76-78]. Few can be removed due to their solubility or chelation chemistry abilities, but the majority gets accumulated either in the body or in the food chain, contributing to a chronic load [76-78].
Not only metal toxicity should be known but also metal speciation needs to be addressed [79]. Many scientific works and books have reported some chelating agents to suppress metal ions load from the body, but the air, soil and water are continuously getting increased in this load [57,80-82]. The uptake of heavy metal ions from soils to edible plants - especially vegetables - and the concentration of them in roots, leaves and fruits can be averagely stated as more concentrated in the leaves, followed by the roots and the least is found in the fruits [23,83]. The chemistry of chelation or coordination chemistry can provide many chelating agents to discharge the load of everyday life of toxic metal ions. Many of them are present in medicinal plants and should be consumed without restrictions. The process of DETOX that the human body does in order to get rid of unused or toxic substances are very complex and needs many chemical substances in a three-phase process. It is not going to be detailed in this review, but the chelating abilities considering acid and base concepts will be briefly explained.
Ralph Gottfrid Pearson, from the University of California, from the 1960’s published his Tirst papers on the chemistry of Hard and Soft Acids and Bases - HSAB - a theory that is fundamental to the understanding of Coordination Chemistry and consequently, to the Biochemistry of uptake and eventual poisoning of exogenous substances (xenobiotics) in human beings [84,85]. Pearson did not publish many scientific papers, but as a clever and complete researcher, the ones he published have since revolutionized the chemistry of acids and bases. The principles of the HSAB theory have many applications in predicting the path of reactions, explaining the stability of complexes in aqueous media, among other things. The scale of hardness and softness was made up considering the size of ionic radii, the electronegativity, capability of being polarized, the presence of a Tilled, half-Tilled and an empty orbital existing at a higher or lower energy level [84,86]. Pearson classified the hard and soft acids (HSAB theory) as described briefly as follows: a) hard acids have small size, are not easy polarizable, presents a high positive charge; b) hard bases are the ones having small ionic radii, high electronegativity, low polarizability. Soft acids c), on the other hand, have larger sizes, are highly polarizable, have low positive charge; d) soft bases also present larger sizes, low electronegativity, high polarizability.
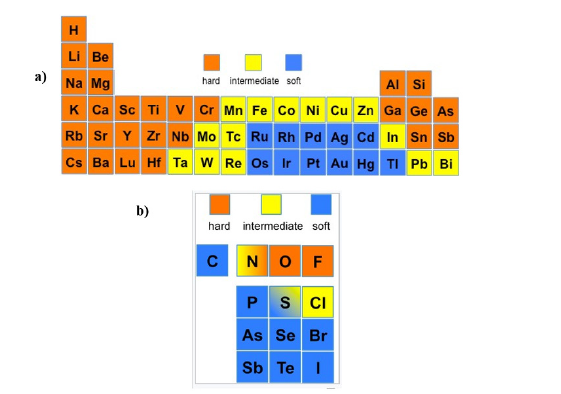
The most stable complexes are those formed between a hard acid and a hard base or between a soft acid and a soft base due to best matches in the chemical features described above. Other combinations can certainly be found but presenting less stability [84]. Among some of the elements described herein, chromium III and VI, uranium IV, manganese II, plutonium IV are hard according to the HSAB theory, and thallium I and III are soft, as well as Selenium+ (RSe+ where R=alkyl). Zinc II are borderline, meaning depending on the conditions, can present hard and/or soft characteristics [84,85]. For the bases, or electron donor compounds, presenting oxygen, in general, makes them hard bases and presenting sulfur or nitrogen in their structures, makes them soft bases [84]. Refer to Figure 12.
Figure 12:a) Soft, intermediate and hard trends for acid behaviour elements and b) soft, intermediate and hard trends for base behaviour elements in the periodic table according to the HSAB theory. (Courtesy of Wikimedia commons). In Table 1 in reference [17], one can Tind two very important properties of the metals ions studied in the review - ligand binding properties, and mobility in aqueous media.
Chelating Substances for Heavy Metal Ions in Medical Treatment and in Environment Remediation
Synthetic substances
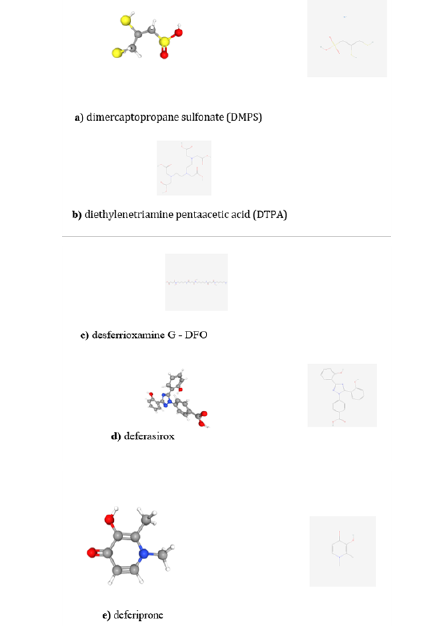
Chemical synthetic substances are available when life is in danger. These substances can bind to the toxic metal ions and make the best of human biochemical system get them out of the living tissues by the Tinal excretion processes the body’s intelligence possess to detox itself. Some examples (refer to Figure 13) can be cited for the toxic metal ions considered in this work. For polonium-a) Dimercaptopropane sulfonate (DMPS) - and for plutonium-b) Diethylenetriamine Pentaacetic Acid (DTPA) [82]; for chelation of thallium, two synthetic substances are known to successfully do so, c) Deferoxamine G (DFO), d) Deferasirox (4- [3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]-benzoic acid) [49] and the Prussian blue salt ([ferric-hexacyanoferrate (II)] ) [49,87]; for chromium (Cr(VI)) d) Deferasirox as well as for thallium, and e) deferiprone (3-hydroxy-1,2-dimethylpyridin-4(1H)- one) [88]; for uranium, depleted or not, polyamine carboxylic acids, the most representative chelating agents, consist of b) diethylenetriaminepentaacetic acid (pentadic acid, DTPA) [57]. Two articles, a 2015 review on chelation therapy for uranium showed some chelating agents for decontamination of this metal ion worth noticing [89] apart from those described above, and another study on the toxicological and detoxification of depleted uranium exposure provides some useful information [57].
A matter of great importance is the study of substances capable of decontaminating soils and waters. Zeolites and other remediating materials have been extensively studied as capable of complexing metal ions in waters and soils. The obtained complex material can then be processed to recover the metals for a better use. Other naturally and slightly modified resources that can perform the same chemical process are bentonite, biochar, soy waste biomass activated by sulfuric acid - the most ecofriendly; and the less ecofriendly - carbon nanomaterials, although more efficient but at a higher cost for living tissues [90]. For many years the group headed by the author of this review studied, synthesized, complexed and modified many natural materials transforming them into a potential metal complex agent in water, wastewaters and soils.
The synthesis included encapsulated binary compounds made of cyclodextrins and a second host for the chelation of metal ions, either essential or toxic, obtaining after the chelation reaction ternary complexes showing good, intermediate or poor stability, resulting in the gradient of viability of their use either to detox humans and / or to remediate wastewaters from various metal ions [91-94]. All these substances (Figure 13), supposed to act as detoxifiers, present the soft character as bases (presence of nitrogen and Sulphur atoms) in order to preferentially chelate regarding the enzymes and proteins in the human living system, the toxic metal ions in an improper concentration. Considering Pearson’s HSAB theory, their synthesis was directed according to these principles. For the essential elements described in this work, as they are only toxic in concentrations greater than the ones the human body can deal with, detox protocols with ingesting plenty of water will suffice in many cases. The alternative protocols for excess of essential metals are out of the scope of this review.
Figure 13:Planar and 3D representation of the chemical structures of a) dimercaptopropane sulfonate (DMPS) [98]; b) diethylenetriamine pentaacetic acid (DTPA) [99]; c) desferrioxamine G [100]; d) deferasirox (4-[3,5-bis(2- hydroxyphenyl)-1,2,4-triazol-1-yl]- benzoic acid) [101]; e) deferiprone [102]. C= gray ball; H=white ball; S=yellow ball; O=red ball; N=blue ball and sticks are bonds among the elements; two sticks in the same direction, represents a double bond between two atoms. All images courtesy of PubChem.
Natural substances for detoxification by chelation of heavy metal ions
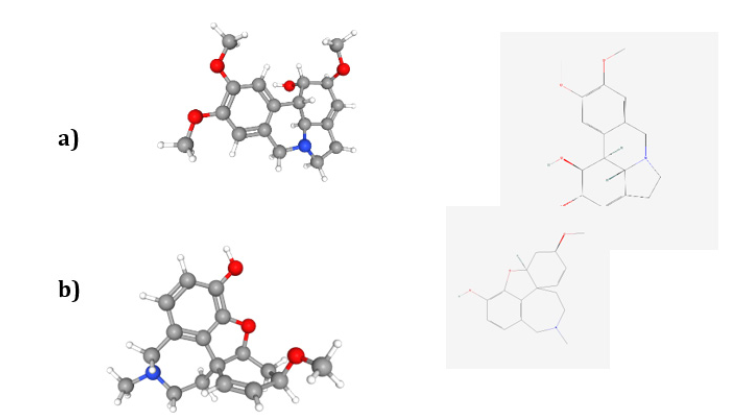
Human endogenous antioxidant systems comprise a series of non-enzyme vitamins (A,C and E), glutathione and Phytochelatins, Flavonoids… metal ions like zinc, copper, manganese, selenium… and enzymes-catalase, glutathione peroxidase and glutathione reductase, superoxide dismutase, catalase among others [95]. Phytochelatins synthase, mainly present in the vegetal world - plants, fungi, some algae and in nematodes, produces biochemically Phytochelatins from glutathione, cysteine and glycine. They are very crucial in the detoxification of heavy metals, acting as master heavy metal chelators in the living realms. Foods of the genus Allium (vegetables, spices that are highly medicinal - botanical family Amaryllidaceae - examples: onion and scallion (Allium cepa L.), garlic (Allium sativum L.), shallot (Allium cepa var. aggregatum), leek (Allium porrum L.), chives (Allium sphenogram L.), wild garlic (Allium Ursinus L.) (ail des ours in France)) contain organosulfur compounds (- trisilicates - soft bases) - chelators of heavy metal ions (soft acids) in the liver and also effective scavengers of hydroxy radicals, singlet oxygen and in lipid peroxidation. The precursor of the trisilicates is S-alk(en)yl-L-cysteine-S-oxide, the whole biosynthetic route starting with the amino acid cysteine and glutamic acid. Allium plants are also reputed for their antibacterial, antifungal and antioxidant properties [96,97]. The is quinoline alkaloids, biogenetically derived from phenylalanine and tyrosine, presenting an is quinoline or a tetrahydroisoquinoline ring (containing a N atom but also -OH- featuring as a soft site base when N is not completed bonded (refer to Figure 14), but mainly a hard one, through the -OH group present in the rings), are in the Amaryllidaceae family, galantamine, lycorine, galantine, haemantamine, calycanthine where the most probable binding site is the -OH groups. Studies showed that these alkaloids are good chelators towards iron(III), also showing a reducing action towards other metal ions. Either way, the detox in a human body is accomplished. Galantamine is an Alzheimer’s disease treatment approved drug [97,98].
Figure 14:2D and 3D representation of the structure of a) galanthine - IUPAC name: (1S,14S,15S,16S)-4,5,14- trimethoxy-9-azatetracyclo[7.6.1.02,7.012,16] hexadeca-2,4,6,12- tetraen-15-ol [107]; and b) chlidanthine- IUPACname:(12S,14R)-14-methoxy-4-methyl-11-oxa4azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15- tetraen-9-ol [108]. C=grey ball; N= blue ball; H=white ball; O=red ball. Courtesy of PubChem [107,108].
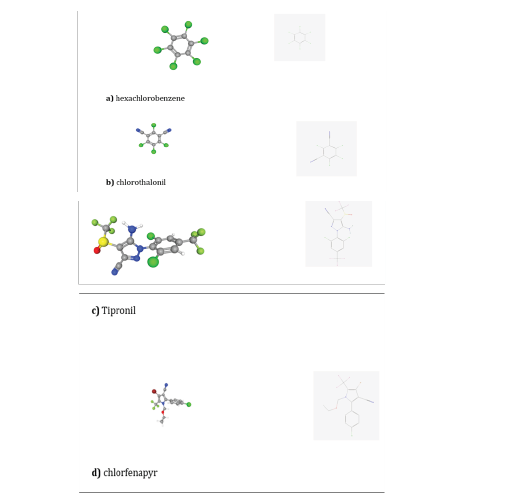
When Allium plants are not cultivated according to the organic principles, some examples of the pesticides that can be used in garlic crops, for instance in China are (refer to in Figure 15): a) hexachlorobenzene, b) chlorothalonil, c) Fipronil and d) chlorfenapyr, all organochloride and heterocyclic insecticides of high persistency in the environment (POPs). Hexachlorobenzene is reasonably anticipated to be a human carcinogen based on association between thyroid, liver and kidney cancer and oral exposure in animals; dangerous - when heated to decomposition, emits toxic fumes of hydrogen chloride; chemically very stable, even to acids and bases [99]. Chlorothalonil when heated to decomposition, emits very toxic fumes of hydrogen chloride, nitrogen oxides, and hydrogen cyanide; It is stable to ultraviolet radiation [100]. Fipronil is a phenyl pyrazole insecticide commonly used in residential and agricultural applications, eliciting neurotoxicity via interactions with GABA and glutamate receptors [101]. Fipronil is reported for downregulating the micro-RNA 155 (miR-155); although this miRNA is small noncoding RNAs, the regulation of this miR-155 has a predicted target gene - cyb561d2 - which being part of cytochrome b561 (cyt b561) family, is involved in electron transfer, cell defense, and chemical stress. A study carried out with Fipronil on miR-155 and cyb561d2 in zebrafish showed that when the expression of miR-155 was downregulated, cyb561d2 was upregulated in both mRNA and protein level in a dose- dependent manner upon stimulation of Fipronil [102].
Figure 15:2D and 3D representation of the structures of a) hexachlorobenzene - IUPAC name: 1,2,3,4,5,6-hexachlorobenzene [109]; b) chlorothalonil - IUPAC name: 2,4,5,6- tetrachlorobenzene-1,3-dicarbonitrile [110]; c) Tipronil - IUPAC name: 5-amino-1-[2,6- dichloro-4-(triTluoromethyl)phenyl]-4-(triTluoromethylsulTinyl) pyrazole-3-carbonitrile [111]; d) chlorfenapyr - IUPAC name: 4-bromo-2-(4-chlorophenyl)-1-(ethoxymethyl)-5- (triTluoromethyl)pyrrole-3-carbonitrile [113]. C=grey ball; Cl=green ball; N=blue ball; S=yellow ball; H=white ball; O=red ball; F=light green ball; Br=violet ball. Courtesy of PUBCHEM.
So, Fipronil is not only known for downregulating the miR- 155, for undergoing environmental degradation to very similar structures that have the same or worse potential toxicity, but also for having a chemical structure never seen in any kind of life form on Earth. It is such a messy and aberrant structure (as all perfluorinated synthetic chemicals) that no enzyme would ever be able to decompose. As this substance is such a bad combination of atoms, no wonder the studies carried out with it showed that this broad-spectrum N- phenyl pyrazole insecticide, with gammaaminobutyric acid type A receptor inhibitory action, causes hyperexcitability of central nervous system. Prior administration of vitamin E or vitamin C in Fipronil exposed mice led to a decrease in lipid peroxidation and significant increase in activities of antioxidants as glutathione, total thiol, superoxide dismutase and catalase. That is to say, the oxidative stress in the studied mice were higher due to this pesticide demanding the ROS defensive mechanism in the rodents to trigger the body defense mechanisms [101].
Chlorfenapyr is a chemical compound of cyanide and a pesticide derived from a class of microbially produced compounds known as halogenated pyrroles. Its use is regulated in most areas due to its toxicity. As chlorothalonil, chlorfenapyr when heated to decomposition emits toxic vapors of hydrogen chloride, hydrogen Fluoride, and nitrogen oxides. Organic nitriles are converted into cyanide ions through the action of cytochrome P450 enzymes in the liver. Cyanide is rapidly absorbed and distributed throughout the body and is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase, but at liver cells cost [103]. The active metabolite uncouples oxidative phosphorylation in the mitochondria causing cellular death. Organic nitriles decompose into cyanide ions both in vivo and in vitro. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted, and the cell can no longer aerobically produce ATP for energy. Another huge toxic consequence is that cyanide binds to the iron (III) in the methemoglobin to form inactive cyanmethemoglobin [103].
Organohalides, especially perfluorinated compounds (made of one or more halogens) are very toxic to mitochondria, studies in vitro and in vivo showed [104]. No need to emphasize one more time the need to consume only organic foods. Milk thistle (Silybum marianum (L.) Garten.), contains Tolazoline substances such as silybin (mixture of 2 diastereomers A and B - Figugre 10), sacristan, sildenafil and 2,3 dehydrin derivatives, all of them reduces oxidative and lipid pro-oxidative damages. When consumed with vitamin C, studies showed in rats, a more effective detoxification of their livers from lead(II) [105]. Silybin (hard oxygen site base) also reduces liver function impairments and has a great chemical affinity to bind iron(III) (hard acid) even at acidic pH, as one can tell by looking at their O atom structure derived Flavonoid. This characteristic puts silybin as a very promising alternative to the synthetic Deferoxamine (DFO) (refer to Fig. 7c and section “Chelating substances for heavy metal ions medical treatment and environment remediation”) as iron-binding ligand. DFO has multiple side effects that the natural silybin certainly does not have (bone deformation, sensory impairment and toxicity) [96]. Cruciferous vegetables, such as broccoli, cauliflower, cabbage, kale, Brussels sprouts, turnips, and kohlrabi are rich sources of sulfur-containing substances [96].
These sulfur-compounds will tend to bind soft metal ions and making the detox of many of the soft heavy metal ions easier. Bioactive and nutritive compounds in the Brassicaceae family are numerous, worth mentioning the glucosinolates and derivatives, isothiocyanates, indoles and phenolic compounds, Flavonoids and anthocyanins. This awesome combination of natural chemical secondary metabolite compounds makes these edible vegetables highly antioxidant, among other amazing properties. One example of the benefits of consuming these sprouts are: lower cancer risks - by the Flavonoids quercetin, kaempferol, cyanidin and Flavonoid glycosides; also by the phenolic acids they biosynthesize: ferulic, caffeic, synaptic, chlorogenic and p-coumaric acids, and their derivatives, to name a few [106] (also refer to Table 1 of reference [106]).
Cilantro / coriander (Coriandrum sativum L.) has two edible parts - stems and leaves and secondly, seeds [96]. The cold pressed oil of the coriander seeds presents linalool, (E)-2- decanal, γ-terpinene, D-limonene, camphor… (average major constituents), and also can present sugar, alkaloids, Flavonoids, resins, tannins, anthraquinones and sterols. The leaves present ferulic, caffeic, garlic and chlorogenic acids. Both leaves and seeds secondary metabolites, present a myriad of different pharmacological activities: anticancer, antioxidant, antimicrobial, antihyperglycemic, anti-hyperlipidemic, anti-spasmodic and liver protectors. So, like the cruciferous, they are very prone to detox human bodies from heavy metals and increase the general homeostasis [107]. Gingko (Ginkgo biloba L.) leaves extract exhibits anti-oxidant properties, scavenging free radicals in different living organs providing protective benefits against sensory processing disorder (neurological condition affecting the brain processing sensory information). As many pesticides have serious neurological impairment, gingko can help ameliorate these kinds of damaged cognition functions. It is known for its beneficial effects in lead poisoning, for reducing oxidative stress, including lipid peroxidation, and promotes elevation of glutathione concentration levels [96].
Conclusion
The edible vegetables and Traditional Chinese Medicinal used and prescribed plants (some cited - cilantro, celery and spinach) that are most susceptible to detox the cells from heavy metals have a double edge sword capacity on them - if we consume them already contaminated with these toxic substances we will increase our problem. Their complex ability is due to their chelating capacity towards soft metal ions and this capacity is not sensitive to the means we would search on them. The plants do not know the difference if they are up taking them and contaminating themselves, or if they are to decontaminate heavy metal charged human living cells as a detoxifying agent [23,108]. From the depleted uranium environmental pollution [109] and polonium and plutonium [110] to the acetylcholinesterase inhibitor pesticides [111] we humans have much to learn that we have the free will to say no to consuming or condoning with their derived goods for profit only. Nature is wiser, not made to deal with human irresponsible acts. However, only human beings have the discernment and the free will to invest and take in their own hands their health and longevity. This review presents some solutions to those that want to start this health and detox path. Make your choice. Following suit is a review on one complete human system detox [112-118].
Acknowledgement
The author thanks Federal University of Paraná - UFPR, and Concelho Nacional de Disinvolvement Cientı́Tico e Tecnológico - CNPq, Brazil.
Conflict of Interest
The author declares that none whatsoever Financial interest or conflict of interest exists.
References
- Deka AK, Talukdar A, Deka DC (2022) Heavy metal monitoring tactics for associated human health risks decline in foods & beverages. Current Nutrition & Food Science 18(8): 690-691.
- Garner CD, Arber JM (1989) X-ray absorption spectroscopy studies of varnado-enzymes nitrogenase and bromo peroxidase. In: Sweet RM, Woodhead AD (Eds.), Synchrotron radiation in structural biology-basic life sciences. Plenum Press, USA, pp. 51.
- Kneifel H, Bayer E (1986) Stereochemistry and total synthesis of amavadin, the naturally occurring vanadium compound of Amanita Muscaria. J Am Chem Soc 108(11): 3075-3077.
- Scherer P (1988) Vanadium and molybdenum requirement for the tixation of molecular nitrogen by two Methanosarcina Archives of Microbiology 151: 44-48.
- Spencer BH, Vanderlelie JJ, Perkins AV (2015) Essentiality of trace element micronutrition in human pregnancy: A systematic review. J Preg Child Health 2(3): 157.
- (2023) Chromium.
- Irving H, Williams RJP (1953) The stability of transition-metal complexes. J Chem Soc, pp. 3192-3210.
- Hougland JL, Kravchuk AV, Herschlag D, Piccirilli JA (2005) Functional identification of catalytic metal ion binding sites within RNA. PLOS Biology 3(9)e277: 1537-1548.
- Laity JH, Lee BM, Wright PE (2001) Zinc finger proteins: New insights into structural and functional diversity. Curr Opin Struct Biol 11(1): 39-46.
- Silva EO, Bracarense APFRL (2016) Phytic acid: From antinutritional to multiple protection factor of organic systems. J Food Sci 81(6): R1357-R1362.
- Maret W (2013) Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv Nutr 4(1): 82-91.
- Maret W (2019) Regulation of cellular zinc ions and their signaling functions. In: Fukada T, Kambe T (Eds.), Zinc signaling. Springer, Singapore, pp 5-22.
- Roohani N, Hurrell R, Kelishadi R, Schulin R (2013) Zinc and its importance for human health: An integrative review. J Res Med Sci 18(2): 144-157.
- Clemens S (2022) The cell biology of zinc. J Exp Bot 73(6): 1688-1698.
- Natasha N, Shahid M, Bibi I, Iqbal J, Khalid S, et al. (2022) Zinc in soil-plant-human system: A data-analysis review. Sci Tot Environ 808: 152024.
- Rondanelli M, Faliva MA, Peroni G, Infantino V, Gasparri C, et al. (2021) Essentiality of manganese for bone health: An overview and update. Natural Product Communications 16(5): 1-8.
- Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, et al. (2022) Essential metals in health and disease. Chem Biol Interact 367: 110173.
- Alejandro S, Höller S, Meier B, Peiter E (2020) Manganese in plants: From acquisition to subcellular allocation. Front Plant Sci Sec Plant Nutr 11: 300.
- Nielsen FH (2001) Boron, manganese, molybdenum and other trace elements. In: Bowman BA, Russell RM (Eds.), Present knowledge in nutrition. 8th (edn.), ILSI Press, USA, pp. 384-400.
- Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Molecular Aspects of Medicine 26(4-5): 353-362.
- Dring JC, Forma A, Chilimoniuk Z, Dobosz M, Teresiński G, et al. (2022) Essentiality of trace elements in pregnancy, fertility, and gynecologic cancers-a state-of-the-art review. Nutrients 14(1): 185.
- Bánfalvi G (2011) Heavy Metals, trace elements and their cellular effects. In: Banfalvi G (Ed.), Cellular effects of heavy metals. Springer, USA.
- Meng M, Yang L, Wei B, Cao Z, Yu J, et al. (2021) Plastic shed production systems: The migration of heavy metals from soil to vegetables and human health risk assessment. Ecotoxicol Environ Safety 215: 112106.
- Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 9(3): 42.
- Mansour SA, Belal MH, Abou AAAK, Gad MF (2009) Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere 75(5): 601-609.
- Dogheim SM, Ashraf EMM, Alla SAG, Khorshid MA, Fahmy SM (2004) Pesticides and heavy metals levels in Egyptian leafy vegetables and some aromatic medicinal plants. Food Addit Contam 21(4): 323-330.
- Jayaraj R, Megha P, Sreedev P (2016) Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol 9(3-4): 90-100.
- Manzoor J, Sharma M, Wani KA (2018) Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J Plant Nutr 41(13): 1744-1763.
- Yang R, Yao T, Xu B, Jiang G, Xin X (2007) Accumulation features of organochlorine pesticides and heavy metals in Tish from high mountain lakes and Lhasa River in the Tibetan Plateau. Environ Int 33(2): 151-156.
- Bordajandi LR, Gómez G, Fernández MA, Abad E, Rivera J, et al. (2003) Study on PCBs, PCDD/Fs, organochlorine pesticides, heavy metals and arsenic content in freshwater Tish species from the River Turia (Spain). Chemosphere 53(2): 163-171.
- Defarge N, Vendômois JS, Séralini GE (2018) Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol Rep 5: 156-163.
- Mesnage R, Ferguson S, Brandsma I, Moelijker N, Zhang G, et al. (2022) The surfactant co-formulant POEA in the glyphosate-based herbicide rangerpro but not glyphosate alone causes necrosis in Caco-2 and HepG2 human cell lines and ER stress in the ToxTracker assay. Food Chem Toxicol 168: 113380.
- Goel A, Aggarwal P (2007) Pesticide poisoning. Nat Med J India 20(4): 182-191.
- Singh N, Gupta VK, Kumar A, Sharma B (2017) Synergistic effects of heavy metals and pesticides in living systems. Front Chem 5: 70.
- (2023) Chlorpyrifos. National Center for Biotechnology Information.
- Singh R, Sadiq NM (2023) Cholinesterase inhibitors. National Library of Medicine, National Center for Biotechnology Information, Stat Pearls Publishing LLC, USA.
- Tzanetou EN, Karasali H (2022) A comprehensive review of organochlorine pesticide monitoring in agricultural soils: The silent threat of a conventional agricultural past. Agriculture 12(5): 728.
- Shaban NS, Abdou KA, Hassan NEHY (2016) Impact of toxic heavy metals and pesticide residues in herbal products. Beni-Suef University Journal of Basic and Applied Sciences 5(1): 102-106.
- Harris ESJ, Cao S, Littlefield BA, Craycroft JA, Scholten R, et al. (2011) Heavy metal and pesticide content in commonly prescribed individual raw Chinese herbal medicines. Sci Tot Environ 409(20): 4297-4305.
- Dong W, Zhang Y, Quan X (2020) Health risk assessment of heavy metals and pesticides: A case study in the main drinking water source in Dalian, China. Chemosphere 242: 125113.
- Kumar N, Kulsoom M, Shukla V, Kumar D, Pryanka, et al. (2018) Profiling of heavy metal and pesticide residues in medicinal plants. Environ Sci Pollut Res 25(29): 29505-29510.
- Urieta I, Jalón M, Eguileor I (1996) Food surveillance in the Basque country (Spain) II. Estimation of the dietary intake of organochlorine pesticides, heavy metals, arsenic, aflatoxin M1, iron and zinc through the total diet study, 1990/91. Food Addit Contam 13(1): 29-52.
- Blain R (2022) Thallium. In: Nordberg GF, Costa M (Eds.), Handbook on the Toxicology of Metals. 5th (edn), Chapter 32, Academic Press, USA, pp. 795-806.
- Douglas KT, Bunni MA, Baindur SR (1990) Thallium in biochemistry. Int J Biochem 22(5): 429-438.
- (2023) The Pale Horse.
- (2023) The Royal Society of Chemistry.
- Cvjetko P, Cvjetko I, Pavlica M (2010) Thallium toxicity in humans. Arh Hig Rada Toksikol 61(1): 111-119.
- Zou H, Zou S (2023) Advanced thallium toxicity. Practic Neurol 23: 85-87.
- Kazantzis G (2007) Thallium. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT (Eds.), Handbook on the toxicology of Metals. 3rd (edn.), Chapter 41, Elsevier, USA, pp. 827-837
- Britten JS, Blank M (1968) Thallium activation of the (Na+K+)-activated ATPase of rabbit kidney. Biochim Biophys Acta 159(1): 160-166.
- Favari L, Mourelle M (1985) Thallium replaces potassium in activation of the (Na+, K+)-ATPase of rat liver plasma membranes. J Appl Toxicol 5(1): 32-34.
- Zhu B, Liang C, Yan S, Li Z, Huang K, et al. (2019) Association between serum thallium in early pregnancy and risk of gestational diabetes mellitus: The Ma’anshan birth cohort study. J Trace Elem Med Biol 52: 151-156.
- Chang P, Kim KW, Yoshida S, Kim SY (2005) Uranium accumulation of crop plants enhanced by citric acid. Environ Geochem Health 27(5-6): 529-538.
- Salminen-Paatero S, Paatero J (2021) Transfer of natural radionuclides in terrestrial food chains-A review of investigations in Finland. Int J Environ Res Public Health 18(20): 10577.
- Santos EE, Lauria DC, Amaral ECS, Rochedo ER (2002) Daily ingestion of 232Th, 238U, 226Ra, 228Ra and 210Pb in vegetables by inhabitants of Rio de Janeiro City. J Environ Radioact 62(1): 75-86.
- Oliver IW, Graham MC, MacKenzie AB, Ellam RM, Farmer JG (2008) Depleted uranium mobility across a weapons testing site: isotopic investigation of porewater, earthworms, and soils. Environ Sci Technol 42(24): 9158-9164.
- Yue YC, Li MH, Wang HB, Zhang BL, He W (2018) The toxicological mechanisms and detoxification of depleted uranium exposure. Environ Health Prev Med 23: 18.
- Zhang Z, Tang Z, Liu Y, He H, Guo Z, et al. (2023) Study on the ecotoxic effects of uranium and heavy metal elements in soils of a Uranium mining area in Northern Guangdong. Toxics 11(2): 97.
- Keith S, Faroon O, Roney N (2013) Toxicological profile for Uranium. Agency for toxic substances and disease registry (US), Atlanta (GA).
- Redvers N, Chischilly AM, Warne D, Pino M, Colbert AL (2021) Uranium exposure in American Indian communities: Health, Policy, and the way forward. Environ Health Perspect 129(3): 35002.
- Stasinos S, Zabetakis I (2013) The uptake of nickel and chromium from irrigation water by potatoes, carrots and onions. Ecotoxicol Environ Saf 91: 122-128.
- Hamilton EM, Young SD, Bailey EH, Watts MJ (2018) Chromium speciation in foodstuffs: A review. Food Chem 250: 105-112.
- Xiao W, Zhang Y, Li T, Chen B, Wang H, et al. (2012) Reduction kinetics of hexavalent chromium in soils and its correlation with soil properties. J Environ Qual 41(5): 1452-1458.
- Kazakis N, Kantiranis N, Kalaitzidou K, Kaprara E, Mitrakas M, et al. (2018) Environmentally available hexavalent chromium in soils and sediments impacted by dispersed fly ash in Sarigkiol basin (Northern Greece). Environ Pollut 235: 632-641.
- Caporale AG, Agrelli D, González PR, Adamo P, Alonso JIG (2019) Hexavalent chromium quantification by isotope dilution mass spectrometry in potentially contaminated soils from south Italy. Chemosphere 233: 92-100.
- (2023) Polonium.
- Tso T, Hallden NA, Alexander L (1964) Radium-226 and Polonium-210 in leaf tobacco and tobacco soil. Science 146(3647): 1043-1045.
- Aoun M, Samrani AGE, Lartiges BS, Kazpard V, Saad Z (2010) Releases of phosphate fertilizer industry in the surrounding environment: investigation on heavy metals and polonium-210 in soil. J Environ Sci (China) 22(9): 1387-1397.
- Borylo A, Olszewski G, Skwarzec B (2013) A study on lead (210Pb) and polonium (210Po) contamination from phospogypsum in the environment of Wiślinka (northern Poland). Environ Sci: Processes Impacts 15(8): 1622-1628.
- Flis ZP, Chrzanowski E, Dembinska S (1997) Intake of 226Ra, 210Pb and 210Po with food in Poland. Sci Total Environ 203(2): 157-165.
- Dyer O (2007) More cases of polonium-210 contamination are uncovered in London. BMJ 334(7584): 65.
- Orzeł E, Komosa A, Grządka A (2022) Plutonium distribution in sequentially extracted phases of arable and uncultivated soils. Environ Earth Sci 81: 411.
- Zhang W, Hou X, Zhang H, Wang Y, Dang H, et al. (2021) Level, distribution, and sources of plutonium in the northeast and north China. Environ Pollut 289: 117967.
- Romanchuk AY, Kalmykov SN, Kersting AB, Zavarin M (2016) Behaviour of plutonium in the environment. Russ Chem Rev 85(9): 995.
- Philips SH, Donaher SE, Powell BA, Tharayil N, Martinez NE (2023) The influence of Iron and ligand type on Plutonium uptake in two strains of hydroponically grown corn (Zea mays). Health Phys 124(2): 97-105.
- Pavlı́cková J, Zbı́ral J, Smatanová M, Habarta P, Houserová P, et al. (2006) Uptake of thallium from naturally-contaminated soils into vegetables. Food Addit Contam 23(5): 484-491.
- Einolghozati M, Ghane ET, Khazaei M, Mehri F (2022) The level of heavy metal in fresh and processed fruits: A study meta-analysis, systematic review, and health risk assessment. Biol Trace Elem Res 201(5): 2582-2596.
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2): 60-72.
- Peana M, Pelucelli A, Medici S, Cappai R, Nurchi VM, et al. (2021) Metal toxicity and speciation: A review. Curr Med Chem 28(35): 7190-7208.
- Crichton R (2016) Metal toxicity-an introduction. In: Crichton R, Ward RJ, Hider RC (Eds.), Metal chelation in medicine. Chapter 1, Royal Society of Chemistry, UK.
- Dong L, He Z, Wu J, Zhang K, Zhang D, et al. (2023) Remediation of uranium-contaminated alkaline soil by rational application of phosphorus fertilizers: Effect and mechanism. Environmental Research 220: 115172.
- Aaseth J, Nurchi VM, Andersen O (2021) Clinical therapy of patients contaminated with polonium or plutonium. Curr Med Chem 28(35): 7238-7246.
- Xu L, Lu A, Wang J, Ma Z, Pan L, et al. (2015) Accumulation status, sources and Phyto availability of metals in greenhouse vegetable production systems in Beijing, China. Ecotoxicol Environ Safety 122: 214-220.
- Pearson RG (1968) Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J Chem Educ 45(9): 581-586.
- Pearson RG (1968) Hard and soft acids and bases, HSAB, part II: Underlying theorie. J Chem Educ 45(10): 643-648.
- Pearson RG (1987) Recent advances in the concept of hard and soft acids and bases. J Chem Educ 64(7): 561- 567.
- Saljooghi AS, Babaie M, Mendi FD, Zahmati M, Saljooghi ZS (2016) Chelation of thallium by combining deferasirox and desferrioxamine in rats. Toxicol Ind Health 32(1): 83-88.
- Iranmanesh M, Fatemi SJ, Ebrahimpour R, Balooch FD (2013) Chelation of chromium(VI) by combining deferasirox and deferiprone in rats. Biometals 26(3): 465-471.
- Joksić AS, Katz SA (2015) Chelation therapy for treatment of systemic intoxication with uranium: A review. J Environ Sci Health A Tox Hazard Subst Environ Eng 50(14): 1479-1488.
- Han B, Weatherley AJ, Mumford K, Bolan N, He JZ, et al. (2022) Modification of naturally abundant resources for remediation of potentially toxic elements: A review. J Hazard Mater 421: 126755.
- Fungaro DA, Bertolini TCR, Madalena CP, Delgado AL, Rodrı́guez O, et al. (2013) Cyclodextrin-modified zeolite from fly ash: Synthesis, characterization, and adsorption properties. Twenty-Eighth International Conference on Solid Waste Technology and Management. Filadelfia, PA, USA.
- Winter E, Fungaro DA, Deguchi TGF, Mercé ALR (2022) Coal ash composite modified with beta-cyclodextrin.
- Soares DF, Noseda MD, Felcman J, Khan MA, Bouet G, et al. (2013) Supramolecular assemblies of Al3+ complexes with vitamin D3 (cholecalciferol) and phenothiazine. Encapsulation and complexation studies in b-cyclodextrin. J Incl Phenom Macrocyclic Chem 75: 137-145.
- Mercé ALR, Nicolini J, Khan MA, Bouet G (2009) Qualitative study of supramolecular assemblies of b-cyclodextrin and cholecalciferol and the cobalt (II), copper (II) and zinc (II) ions. Carbohydrate Polymers 77(2): 402-409.
- Irshad M, Chaudhuri PS (2002) Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol 40(11): 1233-1239.
- Mehrandish R, Rahimian A, Shahriary A (2019) Heavy metals detoxification: A review of herbal compounds for chelation therapy in heavy metals toxicity. J Herbmed Pharmacol 8(2): 69-77.
- Benkeblia N, Lanzotti V (2007) Allium thiosulfinates: Chemistry, biological properties and their potential utilization in food preservation. Food 1(2): 193-201.
- Parvin MS, Chlebek J, Hošťálková A, Catapano MC, Lomozová Z, et al. (2022) Interactions of isoquinoline alkaloids with transition metals iron and copper. Molecules 27(19): 6429.
- Hexachlorobenzene (2023) National Center for Biotechnology Information.
- Chlorothalonil (2023) National Center for Biotechnology Information.
- Fipronil (2023) National Center for Biotechnology Information.
- Huang H, Zhang K, Zhou Y, Ding X, Yu L, et al. (2016) MicroRNA-155 targets cyb561d2 in zebrafish in response to fipronil exposure. Environ Toxicol 31(7): 877-886.
- Chlorfenapyr (2023) National Center for Biotechnology Information.
- Denslow ND, Martyniuk CJ (2023) Organohalides. In: Oliveira MR (Ed.), Mitochondria Intoxication, Chap 26. Academic Press, Elsevier, USA, pp. 557-585.
- Wellington K, Jarvis B (2001) Silymarin: A review of its clinical properties in the management of hepatic disorders. Bio Drugs 15(7): 465-89.
- Abellán A, Perles RD, Moreno DA, Viguera CG (2019) Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients 11(2): 429.
- Abascal K, Yarnell E (2012) Cilantro-culinary herb or miracle medicinal plant? Altern Complement Ther 18(5): 259-264.
- Shahrajabian MH, Cheng Q, Sun W (2022) The organic life according to traditional Chinese medicine with anticancer approaches. Curr Nutr Food Sci 18(8): 692-697.
- Briner W (2010) The toxicity of depleted uranium. Int J Environ Res Public Health 7(1): 303-313.
- Zhou L, Wang R, Ren H, Wang P, Cao Y (2023) Detection of Polonium-210 in environmental, biological and food samples: A review. Molecules 28(17): 6268.
- (2023) Cholinesterase inhibitors: Including insecticides and chemical warfare nerve agents. Environmental Health and Medicine Education-ATSDR -Agency for Toxic Substances and Disease Registry.
- (2023) Unithiol. National Center for Biotechnology Information.
- (2023) Pentetic acid. National Center for Biotechnology Information.
- (2023) Desferrioxamine G. National Center for Biotechnology Information.
- (2023) Deferasirox. National Center for Biotechnology Information.
- (2023) Deferiprone. National Center for Biotechnology Information.
- (2023) Galanthine. National Center for Biotechnology Information.
- (2023) Chlidanthine. National Center for Biotechnology Information.
© 2023 Ana Lucia Ramalho Mercé. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






