- Submissions

Full Text
Advances in Complementary & Alternative medicine
Acute Effects of Alpinia galanga Extract on Mental Alertness, Accuracy and Fatigue in Human Subjects: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Study
Mohan Muttanahally Eraiah1, Mamatha Kundapur2, Lincy Joshua3 and Jestin V Thomas3*
1BGS Global Institute of Medical Sciences, India
2Divakars Speciality Hospital, India
3Leads Clinical Research and Bio Services Pvt. Ltd, India
*Corresponding author:Jestin V Thomas, Leads Clinical Research and Bio Services Pvt. Ltd. No.9, 1st Floor Mythri Legacy, Kalyan Nagar, Chelekere Main Road, Bengaluru 560043, Karnataka, India
Submission: May 12, 2023;Published: May 18, 2023

ISSN: 2637-7802 Volume 7 Issue 4
Abstract
Background: Nootropics are products that improve cognitive function and offer consumers solutions to optimise performance at work and play. Alpinia galanga Extract (AGE) has been shown to increase mental alertness in clinical settings.
Aim: We evaluated the acute effect of AGE on mental alertness, accuracy, and fatigue through a randomized, double-blind, placebo-controlled, cross-over clinical study in healthy human subjects.
Methods: Sixty-two adults were randomized to receive either 300mg of AGE or placebo 30 minutes after lunch on Day 1 followed by cross-over treatments on Day 7. The primary objective was to evaluate the effect of AGE on mental alertness and accuracy, as assessed by Symbol Digit Coding test, Shifting Attention test, Stroop Test and Alertness Rating Scale of CNS Vital Signs at baseline, 0.5, 1-, 2- and 5-hours post dose. Further, fatigue and energy levels were measured by Visual Analogue Scale and daytime sleepiness by Epworth Sleepiness Scale as secondary outcomes. Adverse events were monitored for safety assessment.
Results: AGE supplementation showed significant improvements in alertness, reaction time, correct responses, and reduction in errors at several time points over placebo. Further, AGE consumption demonstrated significant increase in subjective feelings of energy and decreased fatigue levels over placebo.
Conclusion: AGE supplementation improves alertness, accuracy, reaction time, and reduces errors in individuals, and may be a reasonable alternative to caffeine for those seeking same-day effects to increase alertness and energy levels.
Keywords:Alpinia galanga; Cognitive impairment; Energy; Mental alertness; Mental fatigue; Nootropics
Introduction
Our daily lives have become increasingly complex as we navigate through a world that is dominated by digital devices and information overflow. The desire to keep up, be mentally sharp and alert, focused, cope with stress and manage with lack of sleep, is indeed at an all-time high. Strategies to support mental alertness and improve mood and energy including through nutrition and dietary supplements are gaining the attention of consumers [1,2]. Caffeine is an extensively used nootropic to help improve alertness, wakefulness in a fatigued state, and increase mental and physical performance [3-8]. However prolonged caffeine use may lead to a range of adverse effects such as insomnia, palpitations, jitters, headaches, fatigue, anxiety, irritability, gastrointestinal upset, etc. which is generally termed as “caffeine crash” [9-16]. Hence there has been a growing demand for caffeine-free plant-derived nootropics that work quickly to help improve mental focus and alertness and reduce fatigue without the side-effects associated with caffeine use [17]. Alpinia galanga also known as galangal or Thai ginger is a medicinal plant that has been extensively used in traditional medicine given its multiple medicinal properties and central nervous stimulant properties [18-22].
The rhizome is the main plant part of A. galanga used since the rhizome harbors most of the phytochemical compounds including flavonoids, phenolic acids and volatile compounds and shown to have biological activities [23]. Interestingly, the rhizome of galangal is also widely used as spice for flavoring foods throughout Asian countries [19,22,24-26]. A. galanga has been reported to improve cognitive performance in humans [27,28]. Compared to other plant extracts, aqueous extract of A. galanga was associated with significant and stable increase in alertness scores from baseline until 5 hours in healthy subjects [29]. Additionally, A. galanga extract by itself or in combination with 200mg caffeine was able to improve mental alertness and improved sustained attention at 3 hours post dose as compared to baseline [30]. Further, molecular data suggests that neurocognitive enhancing property of A. galanga extract is likely mediated by the interaction of its bioactive components with various targets involved dopamine and Acetylcholinesterase (AchE) pathways [27]. The current study further seeks to strengthen the earlier nootropic findings associated with A. galanga extract, such as acute benefits on mental alertness, accuracy, and fatigue without disturbing sleep architecture in healthy young adults using widely used validated testing methods.
Materials and Methods
?Study participants
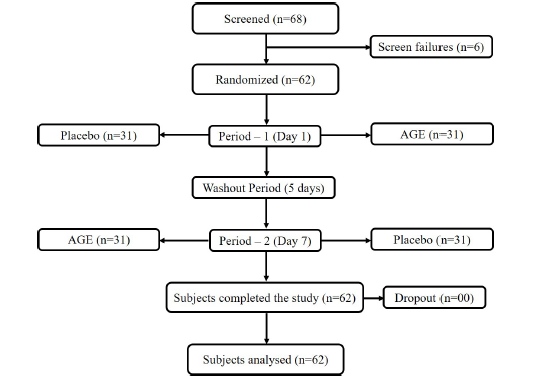
The study was conducted from 01 May 2022 at two centers in India: BGS Global Institute of Medical Sciences, Bengaluru, India and Divakars Specialty Hospital, Bengaluru, India. Sixty-two (62) subjects were enrolled in the study (Supplementary Figure 1). Adult healthy males and/or females, aged between 18 and 55 years who met all inclusion and no exclusion criteria were enrolled in the study after signing a written informed consent. Inclusion criteria were as follows: Body mass index 18.5kg/m2 to 29.9kg/m2, Fatigue Severity Scale score >4, history of consuming <3 cups of tea/coffee per day, post-lunch sleepiness as indicated by Epworth sleepiness score ≥11 and ≤17, agreed to sleep for 8±1 hours the night before the visit day, maintain their usual life-style, agreed to refrain from consuming caffeine and caffeine-containing products 12 hour prior to visit days, agreed to refrain from vigorous physical activity 12 hours prior to visit days, and agreed to stay weight stable during the study period. Demographic details such as date of birth, sex, ethnicity, and race were obtained after the informed consent process. The following criteria were used to exclude subjects in the study: Subjects who had hypersensitivity or history of allergy to the study product, moderate to severe fatigue or having chronic fatigue syndrome, malignant disease or any concomitant end-state organ disease and/or laboratory abnormalities considered by investigators to be risky or that could interfere with data collection, suffering from a metabolic disorder and/or from severe chronic disease with a psychiatric diagnosis other than anxiety or depression, sleep disturbances and/or were taking sleep aid medication, uncontrolled hypertension (systolic blood pressure >160mm Hg or diastolic blood pressure >100mm Hg) at screening, who were on anxiolytics, anti-depressants, antipsychotics, anticonvulsants, antihypertensive, centrally acting corticosteroids, opioid pain relievers, hypnotics, and/or prescribed sleep medications, history of drug and /or alcohol abuse at the time of enrolment. The study was conducted in accordance with ICH-GCP (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use –Good Clinical Practice) guidelines, ‘Declaration of Helsinki’ and Indian Council of Medical Research codes. The study was registered on Clinical Trials Registry-India (CTRI) [Registration No.: CTRI/2022/05/042770] before first subject enrolment. The study was reviewed and approved by Institutional Ethics Committee - BGS Global Institute of Medical Sciences Bengaluru, Indiaand Divakars Speciality Hospital Ethics Committee, Bengaluru, India. Participants provided their written informed consent before enrolment into the study.
Supplementary Figure 1:Consolidated Standards of Reporting Trials (CONSORT) diagram of the trial conduct.
Study design
This was a randomized, double-blind, placebo-controlled, crossover, clinical intervention study. The study was conducted over 3 visits. The first visit (Day -7 to -1) was for screening purposes, wherein subjects meeting inclusion criteria and not meeting exclusion criteria were identified, and written informed consent was obtained. Medical and prior medication history was recorded. Demography details, anthropometric measurements, physical examination, and laboratory assessments were recorded. In the second visit (Day 1-Randomization) subjects eligible for study participation were randomized to receive AGE supplementation or placebo as per the randomization schedule. To eliminate the influence of confounding variables on the study outcomes, participants reported to the study site in the morning of each visit, and efficacy assessments began after lunch (12 to 1.30pm). In addition, both visits were conducted at the same time of day to reduce response variability due to diurnal patterns. During this period, no additional food or beverages containing calories were provided. During their clinic stay, the participants were permitted to drink as much water as they desired and to relax in a room with a comfortable temperature and unrestricted access to mobile phones and magazines. During this time period, psychostimulant activity was strictly prohibited. Study product administration was done 30 minutes after lunch followed by washout period of 5 days. At the third visit (Day 7), subjects were crossed over to receive AGE or placebo as per randomization schedule. The study product AGE weighed 390mg which included 300mg of proprietary extract of A. galanga (commercially known as enXtra®) and 90mg of microcrystalline cellulose and the placebo weighed 390mg (390mg microcrystalline cellulose). Both the study products were filled in scarlet red colored opaque hard gelatin capsules to maintain the double blindness of the study. Further, the randomization schedule was generated by a non-study assigned, independent personnel based on stratified block randomization using a block of 4 to allocate the subjects to receive either AGE product or the placebo in the ratio of 1:1 on the Day 1 ensuring the treatment balance by using R software.
Efficacy and safety parameters
The primary efficacy endpoint was mental alertness as assessed by Symbol Digit Coding (SDC) test, Shifting Attention Test (SAT), Stroop Test (ST) and Alertness Rating Scale (ARS) of CNS Vital Signs (CVS) from baseline to 0.5, 1-, 2- & 5-hours post supplementation on day 1. Secondary efficacy endpoints included evaluation of fatigue, energy, and vigor scores assessed by Visual Analogue Scale to Evaluate Fatigue Severity from baseline to 6-7 hours post dose on day 1 and daytime sleepiness assessed by Epworth Sleepiness Scale from baseline to 0.5, 1-, 2- & 5-hours post supplementation on day 1. Safety was assessed by monitoring adverse events, physical examination, and vital signs measurement. Baseline assessments were done after lunch before study product consumption using SDC, SAT, ST, ARS, and Epworth Sleepiness Scale (ESS) from CNS VS tool and Visual Analogue Scale to Evaluate Fatigue Severity (VAS-F) for fatigue, energy and vigor scores. Study product administration was done as per the randomization chart 30 minutes after lunch followed by assessments for SDC, SAT, ST, ARS, and ESS measures using CNS VS tool at 0.5, 1-, 2- and 5-hours post dose and VAS-F at end of Day 1 assessment (6-7 hours post dose) and adverse event monitoring. A window of ±5 minutes was allowed from the time points. The same schedule was followed on day 7 after cross-over of study participants and products.
Efficacy endpoints evaluation
CNS-VS assessments are extensively validated and robust computerized neuropsychological tests that measure neurocognitive status of subject. CNS-VS encompasses a range of mental processes from simple motor performance, attention, memory, to executive functions with >50 clinical and quality measures [6]. In the current study, SDC, SAT, ST, ARS, and ESS were assessed as a part of CNSVS panel. The mean correct responses in the study were calculated by averaging the correct responses data from SDC and SAT, mean errors was calculated by averaging data from SDC, SAT and ST; and mean correct reaction time was calculated by averaging data from SAT and ST. Symbol Digit Coding (SDC) test provides scores for correct responses and errors and covers domains such as complex information, processing accuracy, complex attention, visualperceptual speed, and information processing speed. Shifting Attention Test (SAT) provides scores for correct responses, errors and correct reaction time and covers domains such as executive function, shifting sets: rules, categories and rapid decision making and reaction time. Stroop Test (ST) provides scores for simple reaction time, complex reaction time correct, Stroop reaction time correct, Stroop commission errors and covers domains such as simple reaction time, complex reaction time, Stroop reaction time, inhibition/ disinhibition, frontal and executive skills. The visual analogue scale to evaluate fatigue (VAS-F) severity uses 18 items relating to the subjective experience of fatigue validated for adults aged 18-55 years. Each item requires respondents to mark an “X,” representing their current state of mind, on a visual analogue scale extending between two extremes (e.g., from “not at all tired” to “extremely tired”). Each line is 100mm in length; consequently, scores range from 0 to 100. The instrument also has two subscales: fatigue (1-5 and 11-18 items) and vitality (6-10 items). Vigor subscore was taken from the response to question no. 8. The ESS is an 8-question self-administered questionnaire. Respondents are asked to rate their normal chances of dozing off or falling asleep while engaged in eight different activities on a 4-point scale (0-3). The ESS score (the sum of 8 item ratings ranging from 0 to 3) can vary from 0 to 24. The higher the ESS score, the greater the person’s average sleep propensity in everyday living (ASP), often known as ‘daytime sleepiness’.
Statistical analysis
Assuming a common standard deviation of 4.50 for alertness tool at the end of treatment, a total of 52 subjects was considered sufficient to detect a mean difference of 3.00 between the two treatments with power of 90% and a 0.05 two-sided level of significance. A sample size of total of 62 subjects was enrolled assuming 15% drop out at the end of the study. For continuous endpoints, results were summarized using descriptive statistics: number of subjects (n), mean, standard error. For evaluations involving the primary and secondary efficacy endpoints, mean changes from baseline to the end of study were computed. Paired t-test was applied to assess within group analysis. Independent t-test was applied to assess between group analysis for the actual change. A value of p<0.05 was considered statistically significant. Categorical variables were summarized using counts and percentages. The comparisons between the treatment groups with AGE and placebo were evaluated using the Pearson’s Chi-Square test or Fisher’s exact test, as appropriate. The analysis was conducted on the safety population. For inferential tests, p-value<0.05 and 95% confidence intervals was considered for statistical significance and two-tailed hypothesis was tested.
Results
Baseline characteristics
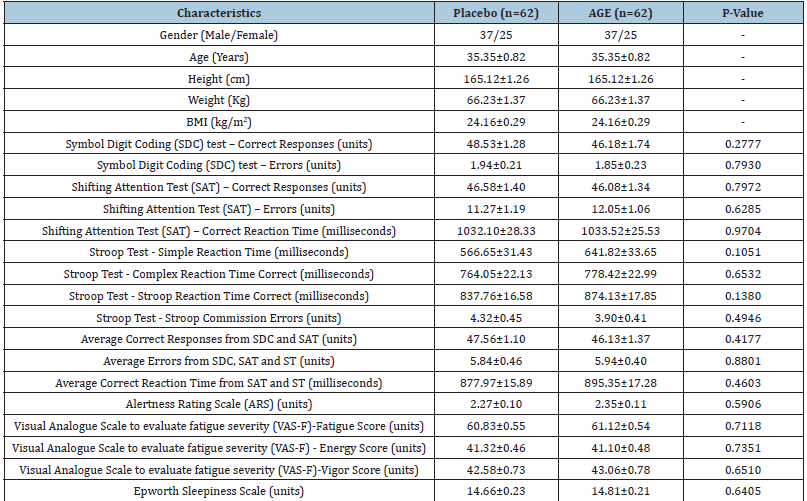
The mean (±SE) age of subjects enrolled in the study was 35.35±0.82 years. The enrolled subjects had mean (±SE) body weight of 66.23±1.37 Kg, height of 165.12±1.26 cm and Body Mass Index (BMI) of 24.16±0.29 Kg/m2. Sixty percent subjects were male, and 40% subjects were female. All subjects in the study were of Indian origin. Baseline characteristics of both the groups did not show any significant difference at baseline (Table 1).
Table 1:Demographics and baseline characteristics of subjects in study groups.
Primary efficacy endpoints analysis
CNS vital signs: SDC test
Figure 1:CNS Vital Signs-Symbol Digit Coding (SDC) test results. a. Correct Responses and b. Errors.
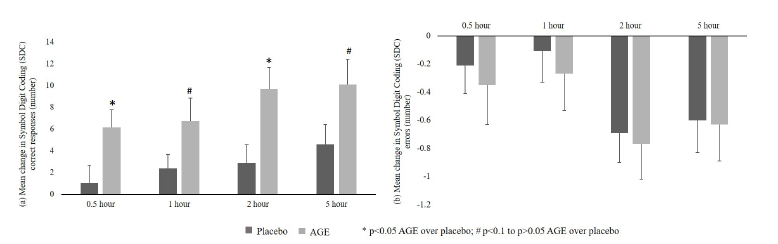
a. Correct responsesA significant (p<0.05) increase in correct responses from baseline was reported in the AGE group as compared to placebo at 0.5 and 2-hour time points. Further, an increasing trend (p<0.1) in correct responses from baseline was seen in AGE group as compared to placebo group at 1 and 5-hour time points (Figure 1a).
b. Errors: No significant difference was reported between AGE and placebo groups in reducing errors from baseline at all the time points post-dose (Figure 1b).
CNS vital signs: SAT test
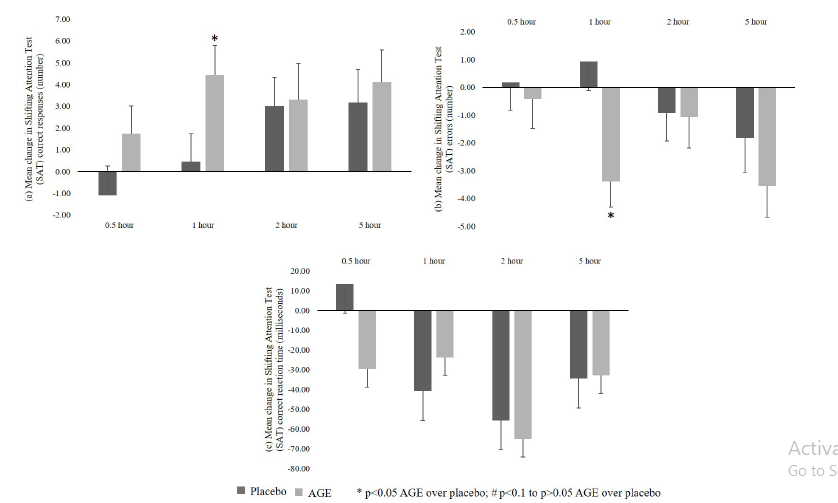
a. Correct responses: A significant (p<0.05) increase in correct responses from baseline was recorded in AGE group compared to placebo group at 1-hour. Further, no significant difference from baseline was seen between groups for other time points at 0.5, 2 and 5-hour (Figure 2a).
b. Errors:A significant (p<0.05) decrease in errors from baseline was recorded in AGE group as compared to placebo group at 1-hour time and no significant decrease was seen between groups at other time points at 0.5, 2 and 5-hour (Figure 2b).
c. Correct reaction time: No significant differences were reported between AGE and placebo groups in correct reaction time from baseline at all the time points post-dose (Figure 2c).
Figure 2:CNS Vital Signs - Shifting Attention (SAT) test results. a. Correct Responses; b. Errors; and c. Correct Reaction Time.
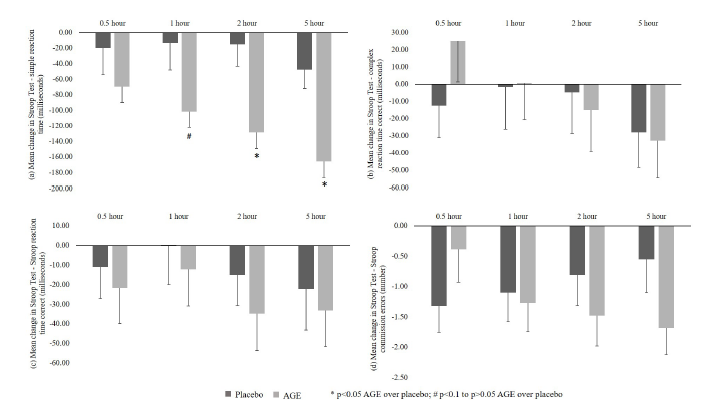
CNS vital signs: Stroop test
a. Simple reaction time:A significant (p<0.05) decrease in simple reaction time from baseline was reported in AGE group as compared to placebo group at 2 and 5-hour time points. Further, a decreasing trend (p<0.1) in simple reaction time from baseline was observed at 1-hour and no significant decrease seen at 0.5-hour time point (Figure 3a).
b. Complex reaction time correct:No significant differences were reported between AGE and placebo groups in complex reaction time correct from baseline at all the time points post-dose (Figure 3b).
c. Stroop reaction time correct: No significant differences were observed between AGE and placebo groups in Stroop reaction time correct from baseline at all the time points post-dose (Figure 3c).
d. Stroop commission errorsNo significant differences were reported between AGE and placebo groups in Stroop commission errors from baseline at all the time points post-dose (Figure 3d).
Figure 3:CNS Vital Signs Stroop Test results. a. Simple Reaction Time; b. Complex Reaction Time Correct; c. Stroop Reaction Time Correct and d. Stroop Commission Errors.
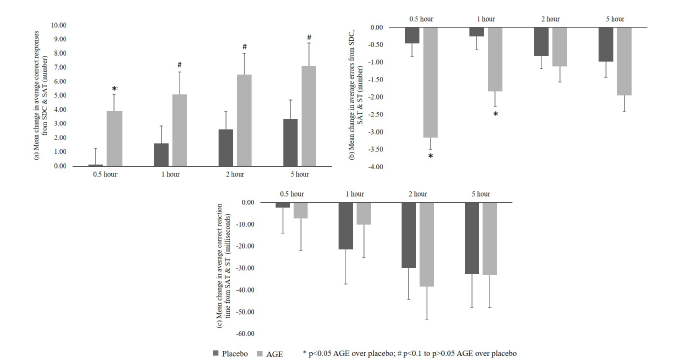
CNS vital signs: Average correct responses from SDC and SAT
Significant (p<0.05) increase in average correct responses from baseline was recorded in AGE group as compared to placebo at 0.5- hour time point post-dose. Further, an increasing trend (p<0.1) in average correct responses from baseline was seen in AGE group compared to placebo group at 1, 2 and 5-hour time points postdose (Figure 4a).
CNS vital signs: Average errors scoring from SDC, SAT and ST
Significant (p<0.05) decrease in average errors from baseline was recorded in AGE group as compared to placebo at 0.5 and 1-hour time points post-dose. Further, no significant difference was recorded between AGE and placebo groups at 2 and 5-hour time points post-dose (Figure 4b).
CNS vital signs: Average correct reaction time from SAT and ST
No significant differences were seen between AGE and placebo groups in average correct reaction time from baseline at all the time points post-dose (Figure 4c).
Figure 4:CNS Vital Signs: a. Average Correct Responses from SDC and SAT; b. Average Errors from SDC, SAT and ST; and c. Average Correct Reaction Time from SAT and ST.
Alertness rating scale
A significant (p<0.05) increase in alertness score from baseline as assessed by ARS was recorded in AGE group as compared to placebo group at 2 and at 5-hour time points post-dose. No significant differences were observed between groups at 0.5 and 1-hour time points (Figure 5a).
Secondary efficacy endpoints analysis
Visual analogue scale to evaluate fatigue severity:
a. Fatigue score: A significant decrease (p<0.05) in fatigue score from baseline as assessed by VAS was recorded in AGE group as compared to placebo group at the end of study (6-7 hours post dose) (Figure 5b).
b. Energy score: A significant (p<0.05) increase in energy score from baseline as assessed by VAS was recorded in AGE group as compared to placebo group at the end of study (6-7 hours post dose) (Figure 5b).
c. Vigor sub-scale of visual analogue scale to evaluate fatigue severity:An increasing trend (p<0.1) in the vigor score from baseline was recorded in AGE group as compared to placebo group at the end of study (6-7 hours post dose) (Figure 5b).
Epworth sleepiness scale:A significant (p<0.05) decrease in daytime sleepiness from baseline as assessed by ESS was recorded in AGE group as compared to placebo at 1, 2 and 5-hour post dose. A decreasing trend (p<0.1) was observed between the groups at 0.5- hour post dose; however, no significance difference was seen either in AGE or placebo groups from baseline (Figure 5c).
Figure 5:a. Alertness Rating Scale results; b. Visual Analogue Scale - Fatigue (VAS-F) severity results; c. Epworth Sleepiness Scale (ESS) results

Adverse events
Three subjects in the study reported adverse events - one (1.61%) AE was experienced with AGE and, 2(3.22%) AEs during placebo administration. Headache was the most common AE experienced by 3(4.84%) subjects; 1(1.61%) during AGE and 2(3.22%) during placebo administration. Common cold was reported by a single subject during placebo administration. All the AEs reported by the subjects were mild in severity and the causality of the AEs was diagnosed by the investigator as not related to the study product. The outcomes of all the AEs were noted as resolved before the end of the study. None of the subjects reported any Serious AE (SAE) or were withdrawn from the study due to an AE or a SAE.
Concomitant medications
Total 3 subjects used at least one concomitant mediation during the course of the study, of which 2 consumed during placebo and 1 during AGE administration. Paracetamol was the most commonly used medication.
Discussion
Previously published reports have demonstrated that the aqueous extract of A. galanga promotes alertness up to five hours post dose. This contrasts with caffeine, which rapidly promotes mental alertness within the first hour followed by a steep decline thereafter [29]. The current study was conducted to further strengthen the existing data on the same day nootropic benefits of AGE using a larger subject population and a combination of validated objective and subjective tools to comprehensively measure the acute effects of AGE on mental alertness and fatigue. We supplemented healthy subjects having mild fatigue with AGE or placebo in a randomized, double-blind, placebo-controlled, crossover study. Our primary objective was to measure mental alertness using the CNS Vital Signs battery of tests followed by fatigue and energy levels through VAS-F questionnaire as secondary objective at 0.5, 1, 2, 3, and 5 hours and 6-7 hours post dose respectively, as compared to baseline. Our results indicate that AGE supplementation significantly improved aspects of mental energy including mental alertness, reaction time, correct responses, reduction in errors and attention, as early as 0.5 hours and sustained until five hours post dose. Further, AGE demonstrated significant increase in subjective feelings of energy and decreased fatigue levels and thus could be an effective alternative to caffeine for individuals looking to improve alertness and attention without suffering from adverse effects of caffeine consumption. AGE was found to be safe without any related adverse events and with no clinically significant changes observed in the vitals throughout the study. Low mental energy at any time of the day is associated with low alertness, attention, focus, vigor, and energy which may subsequently compromise performance on daily tasks and general productivity [31-35]. Consumers are looking for ways to manage their mental energy for optimal performance including through use of plant-based nootropics that are caffeinefree and have no adverse effects [1]. In this regard, aqueous extract of A. galanga has been shown to induce acute, same-day benefits on mental alertness post consumption in healthy human subjects [29]. Our study further validates these earlier findings where we measured the same-day, acute effects of AGE on mental alertness as assessed by battery of tests from CNS Vital Signs and fatigue and energy levels by VAS-F and daytime sleepiness by ESS.
CNS vital signs is a computerized neurocognitive test that objectively measures a broad-spectrum of brain function performance and is extensively used in the clinical setting. The alertness feeling was further supported by significant decrease (p<0.05) in daytime sleepiness as measured by ESS at 1-, 2-, and 5-hour post dose from baseline in AGE group as compared to placebo, as well as measures of increased accuracy and attention. These measures of mental energy were further corroborated by reduced fatigue and increased feelings of vigor and energy in the subjects taking AGE. Mental alertness refers to the state of being watchful with continued attention and ready for quick action [36] whereas mental fatigue is caused by overdrive of the brain leading to exhaustion, hampering cognitive functions and loss of overall productivity [33]. The neural network related to alertness and fatigue is primarily located in the thalamus and bilateral frontal and parietal brain regions with dense dopaminergic innervation [37,38]. Dopamine is also known to increase levels of alertness by blocking adenosine receptors in the forebrain. Caffeine, which is a psychostimulant apart from blocking adenosine receptor, is also thought to up-regulate dopaminergic activity [39]. A previously published docking study [27] indicates that AGE extract increases dopamine levels by blocking dopamine uptake in neuronal synapse as well as influencing acetylcholinesterase activity. Our in-house unpublished biochemical assays also indicate that AGE inhibits enzyme Catechol-O-Methyltransferase (COMT) which is an enzyme responsible for the degradation of dopamine in synaptic space. These preliminary mechanisms of action data indicate that AGE helps to enhance neurotransmitter activity, e.g., dopamine levels in synaptic space and help to improve visuospatial performance and mental alertness. Role of increased dopamine levels along with inhibition glutamate reuptake in improving cognitive abilities has also been demonstrated in case of combination of L-theanine and green tea extract [40,41]. The rhizome of A. galanga comprises a rich phytochemical composition including a wide range of volatile oils associated with free radical scavenging, strong superoxide anion scavenging, metal chelating activity, and anti-inflammatory benefits [26] that may further help in improving cognitive function [42]. The safety of AGE in this study reconfirms previous safety results from clinical studies. Current study is limited for not controlling the factors contributing to fatigue such as social life, work hours, work related stress etc. However, we did standardize subjects entering the study with inclusion criteria for fatigue levels.
Conclusion
The study provides strong support for the acute effect of AGE on improved alertness, accuracy, and attention as early as 30 minutes after supplementation and lasting for up to five hours. This evidence strengthens the findings of previous studies in this area. We believe that AGE could be a viable alternative to caffeine for individuals seeking to increase their alertness, accuracy, and energy levels.
Acknowledgments
We thank the participants of the study.
Fundings
The work was supported by OmniActive Health Technologies Limited (Mumbai, India).
Availability of Data
Data are available from the corresponding author upon a reasonable request.
Author Contributions
MME, MK, JT contributed to the study conception and design. Study conduct, subject recruitment and data collection were performed at respective study centres by MME & MK. Study was monitored in blinded fashion by LJ. Statistical analysis and study report were prepared by JT. Data interpretation was done by MME, MK and JT. The first draft of the manuscript was written by MME, MK and JT. All authors provided their inputs, read and approved the final manuscript.
Consent for Publication
The authors provide their consent for publishing this study results.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee - BGS Global Institute of Medical Sciences Institutional Ethics Committee and Divakars Speciality Hospital Ethics committee, Bengaluru, India (Protocol number LCBS-OA-93).
Supplemental Material
Supplemental material (CONSORT - Diagram) for this article is available.
References
- Dresler M, Sandberg A, Bublitz C, Ohla K, Trenado C, et al. (2018) Hacking the brain: Dimensions of cognitive enhancement. ACS Chemical Neuroscience 3(10): 1137-1148.
- Godos J, Currenti W, Angelino D, Pedro M, Sabrina C, et al. (2020) Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 9(4): 346.
- Gualtieri CT, Johnson LG (2006) Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Archives of Clinical Neuropsychology 21(7): 623-643.
- Haskell CF, Kennedy DO, Wesnes KA, Milne AL, Scholey AB (2007) A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guarana in humans. Journal of Psychopharmacology 21(1): 65-70.
- Kamimori GH, McLellan TM, Tate CM, Voss DM, Niro P, et al. (2015) Caffeine improves reaction time, vigilance and logical reasoning during extended periods with restricted opportunities for sleep. Psychopharmacology 232(12): 2031-2042.
- Kennedy DO, Haskell CF, Wesnes KA, Scholey AB, et al. (2004) Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: comparison and interaction with Panax ginseng. Pharmacology Biochemistry and Behavior 79(3): 401-411.
- Kennedy DO, Haskell CF, Mauri PL, Scholey AB (2007) Acute cognitive effects of standardised Ginkgo biloba extract complexed with phosphatidylserine. Human Psychopharmacology 22(4): 199-210.
- Rigney U, Kimber S, Hindmarch I (1999) The effects of acute doses of standardized Ginkgo biloba extract on memory and psychomotor performance in volunteers. Phytotherapy Research 13(5): 408-415.
- Bernstein GA, Carroll ME, Thuras PD, Cosgrove KP, Roth ME (2002) Caffeine dependence in teenagers. Drug and Alcohol Dependence 66(1): 1-6.
- Burgalassi A, Ramacciotti CE, Bianchi M, Coli E, Polese L, et al. (2009) Caffeine consumption among eating disorder patients: Epidemiology, motivations, and potential of abuse. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity 14(4): e212-e218.
- Ciapparelli A, Paggini R, Carmassi C, Taponecco C, Consoli G, et al. (2010) Patterns of caffeine consumption in psychiatric patients. An Italian study. European Psychiatry 25(4): 230-235.
- Graham K (1988) Reasons for consumption and heavy caffeine use: Generalization of a model based on alcohol research. Addictive Behaviors 13(2): 209-214.
- Indrayan AK, Agrawal P, Rathi AK, Shatru K, Agrawal AS, et al. (2009) Nutritive value of some indigenous plant rhizomes resembling ginger. Natural Product Radiance 8(5): 507-513.
- Juliano LM, Evatt DP, Richards BD, Griffiths RR (2012) Characterization of individuals seeking treatment for caffeine dependence. Psychology of Addictive Behaviors 26(4): 948-954.
- Strain EC, Mumford GK, Silverman K, Griffiths RR (1994) Caffeine dependence syndrome: Evidence from case histories and experimental evaluations. Jama 272(13): 1043-1048.
- Striley CL, Griffiths RR, Cottler LB (2011) Evaluating dependence criteria for caffeine. Journal of Caffeine Research 1(4): 219-225.
- Lorca C, Mulet M, Caro CA, Sanchez MA, Perez A, et al. (2021) Plant-derived nootropics and human cognition: A systematic review. Critical Reviews in Food Science and Nutrition, pp: 1-25.
- Arambewela LS, Arawwawala M, Owen NL, Jarvis B (2007) Volatile oil of Alpinia galanga of Sri Lanka. Journal of Essential Oil Research 19(5): 455-456.
- Chudiwal AK, Jain DP, Somani RS (2010) Alpinia galanga–An overview on phyto-pharmacological properties. Indian Journal of Natural Products and Resources 1(2): 143-149.
- Mahae N, Chaiseri S (2009) Antioxidant activities and antioxidative components in extracts of Alpinia galanga (L.) Sw. Agriculture and Natural Resources 43(2): 358-369.
- Singh JH, Alagarsamy V, Diwan PV, Kumar SS, Nisha JC, et al. (2011) Neuroprotective effect of Alpinia galanga (L.) fractions on Aβ (25-35) induced amnesia in mice. Journal of Ethnopharmacology 138(1): 85-91.
- Yang X, Eilerman RG (1999) Pungent principal of Alpinia galangal (L.) Swartz and its applications. Journal of Agricultural and Food Chemistry 47(4): 1657-1662.
- Ghosh S and Rangan L (2013) Alpinia: the gold mine of future therapeutics. 3 Biotech 3(3): 173-185.
- Jain AP, Pawar RS, Lodhi S, Singhai AK (2012) Immunomodulatory and anti-oxidant potential of Alpinia galanga Linn. rhizomes. Phcog Commn 2(3): 30-37.
- Jirovetz L, Buchbauer G, Shafi MP, Leela NK (2003) Analysis of the essential oils of the leaves, stems, rhizomes and roots of the medicinal plant Alpinia galanga from southern India. Acta Pharmaceutica-Zagreb- 53(2): 73-82.
- Saha S, Banerjee S (2013) Central nervous system stimulant actions of Alpinia galanga (L.) rhizome: A preliminary study. Indian J Exp Biol 51(10): 828-832.
- Sivanandan S, Pimple S (2018) Molecular docking studies of Alpinia galanga phytoconstituents for psychostimulant activity. Advances in Biological Chemistry 8(4): 69.
- Srivastava S, Pimple S (2017) Effects of cymbopogon flexuosus, Alpinia galanga, and Glycyrrhiza glabra on attention: A randomized doubleblind, placebo-controlled pilot study. BAOJ Nutrition 3: 1.
- Srivastava S, Mennemeier M, Pimple S (2017) Effect of Alpinia galanga on mental alertness and sustained attention with or without caffeine: A randomized placebo-controlled study. Journal of the American College of Nutrition 36(8): 631-639.
- Srivastava S (2018) Selective enhancement of focused attention by Alpinia galanga in subjects with moderate caffeine consumption. Open Access J Clin Trials, pp.43-49.
- Heckman MA, Sherry K, Mejia EGD (2010) Energy drinks: An assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Comprehensive Reviews in Food Science and Food Safety 9(3): 303-317.
- Junghaenel DU, Christodoulou C, Lai JS, Stone AA (2011) Demographic correlates of fatigue in the US general population: Results from the Patient-Reported Outcomes Measurement Information System (PROMIS) initiative. Journal of Psychosomatic Research 71(3): 117-123.
- Li G, Huang S, Xu W, Jiao W, Jiang Y, et al. (2020) The impact of mental fatigue on brain activity: A comparative study both in resting state and task state using EEG. BMC Neuroscience 21(1): 1-9.
- Ricci JA, Chee E, Lorandeau AL, Berger J (2007) Fatigue in the US workforce: Prevalence and implications for lost productive work time. Journal of Occupational and Environmental Medicine 49(1):1-10.
- Figuera SR, McCrone P, Hurley M, King M, Donaldson AN, et al. (2010) The hidden cost of chronic fatigue to patients and their families. BMC health services research 10(1): 1-7.
- Johansson B, Berglund P, Rönnbäck L (2009) Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Injury 23(13-14): 1027-1040.
- Ferré S, Fuxe K, Fredholm BB, Morelli M, Popoli P (1997) Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends in Neurosciences 20(10): 482-487.
- Cabezas MÁG, Rico B, González MÁS, Cavada C (2007) Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage 34(3): 965-984.
- Nathan PJ, Lu K, Gray M, Oliver C (2006) The neuropharmacology of L-theanine (N-ethyl-L-glutamine) a possible neuroprotective and cognitive enhancing agent. Journal of Herbal Pharmacotherapy 6(2): 21-30.
- Kakuda T, Nozawa A, Sugimoto A, Niino H (2002) Inhibition by theanine of binding of [3H] AMPA,[3H] kainate, and [3H] MDL 105,519 to glutamate receptors. Bioscience, Biotechnology, and Biochemistry 66(12): 2683-2686.
- Srividya A, Dhanabal S, Satish Kumar M, Bavadia PKH (2010) Antioxidant and antidiabetic activity of Alpinia galanga. International Journal of Pharmacognosy and Phytochemical Research 3(1): 6-12.
- Meng H, Hale L, Friedberg F (2010) Prevalence and predictors of fatigue among middle-aged and older adults: Evidence from the Health and Retirement study. Journal of the American Geriatrics Society 58(10): 2033-2034.
© 2023 Jestin V Thomas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






