- Submissions

Full Text
Advances in Complementary & Alternative medicine
Chemical Constituents of Palm Kernel Oil
Chinedu Imo* and Ejim Moses Ijagem
Department of Biochemistry, Faculty of Pure and Applied Sciences, Federal University Wukari, Nigeria
*Corresponding author: Dr. Chinedu Imo, Department of Biochemistry, Faculty of Pure and Applied Sciences, Federal University Wukari, P.M.B. 1020, Wukari, Taraba State, Nigeria
Submission: July 12, 2021;Published: December 23, 2021

ISSN: 2637-7802 Volume 7 Issue 1
Abstract
The chemical constituents of palm kernel oil were evaluated in this study. Palm kernel oil is produced traditionally or industrially from the kernel of fruits of tropical palm tree. It is used for different purposes in industries, nutrition and medicine. For the purpose of this study, palm kernel oil was produced traditionally. Its chemical constituents were determined with the use of GC (model No. 7890B) and MS detector (model 5977A). Different constituents as contained in table 1, which possess different properties were detected in the palm kernel oil. Some of the chemical constituents detected in it have been reported to possess deleterious effect when used in excess, while many of the constituents have been reported to possess different important nutritional, industrial and medicinal/pharmaceutical properties. The constituents showed palm kernel oil to be good for industrial, medicinal and nutritional purposes.
Keywords:Chemical constituents; Food additive; Industrial; Nutrition; Palm kernel oil
Introduction
Palm kernel oil is an edible oil extracted from the kernels of the fruits of tropical palm tree (Elaeis guineensis Jacq.) [1,2]. In Nigeria, the process of extraction is mostly carried out locally by de-shelling the palm kernel with the use of a crusher in the inform of moderately heavy object. This helps to separate the kernel from the shell which is then heated in a pot to extract the oil which melts out of the nut into the pot. The oil is then decanted into a clean container and allowed to cool before been used or stored. The palm kernel oil may then be used alone or in combination with other substances/oil for different purposes, such as in traditional medicine or in the production of cocoa-butter substances, confectionary fats, biscuit doughs, filling cream, cake icing, and margarine [3,4].
Palm kernel oil may also be used in the manufacturing of different non-edible products such as soap/detergent, candle, cosmetics/cream, grease/lubricants for machines, printing inks and pharmaceuticals products. It is commonly used in traditional medicine as an antidote for poison and for wound healing [4]. Palm kernel oil has been reported to be underutilized as edible oil in Nigeria. Its use in nutrition as a source of dietary fat was also reported by Sutapa & Analava [5] not to pose risks for coronary artery disease when consumed in realistic amounts. Sutapa & Analava [5] reported that oxidized palm kernel oil may induce reproductive and organ toxicity, especially of the liver, heart, kidneys and lungs.
Imo et al. [4] reported a significant increase (P<0.05) in total proteins, albumin and globulin; a non-significant decrease in ALT and AST activities, and a non-significant increase in ALP activity upon administration of palm kernel oil in male albino rats and predicted that the result could be an indication of non-toxic effect exhibited on the liver cells by the oil, whereas they also reported a significant rise (P<0.05) in bilirubin in the same animals. Due to the wide use of palm kernel oil for various reasons in many parts of the world, it is important to investigate its chemical constituents. The knowledge of its constituents will show various areas of usefulness or application of palm kernel oil.
Materials and Methods
Palm kernel oil used
The palm kernel oil used was produced and obtained from Olokoro in Umuahia, Nigeria.
Determination of chemical constituents of palm kernel oil
The method reported by Imo et al. [6] was used. The chemical constituents of palm kernel oil were determined with the use of GC (model No. 7890B) and MS detector (model 5977A). The GC-MS was equipped with column: Agilent HP 5MS ultra-Inert (350 °C) 30m × 250μm × 0.25μm. Helium with flow: 0.7ml/min was used as the gas with pressure: 4.4867 psi and average velocity of 30.641 cm/seconds. One (1)ml injection volume with inlet temperature of 250 °C, split flow 14ml/min and split ratio 20:1 was used. The oven temperature used was 60 °C with 1min equilibrating time, maximum temperature of 350 °C, and total run time of 35.857min. Chemical constituents of the palm kernel oil were then identified by matching the spectra of the constituents identified with that of the mass spectra of reference compounds contained in the database of National Institute of Standards and Technology (NIST 14). The amount/concentration of the chemical constituents suggested to be present in the palm kernel oil were then expressed as area percent which is comparable to the total peak area.
Results and Discussion
The results of the study are presented in the table and figure below (Table 1).
Table 1:Chemical constituents of palm kernel oil
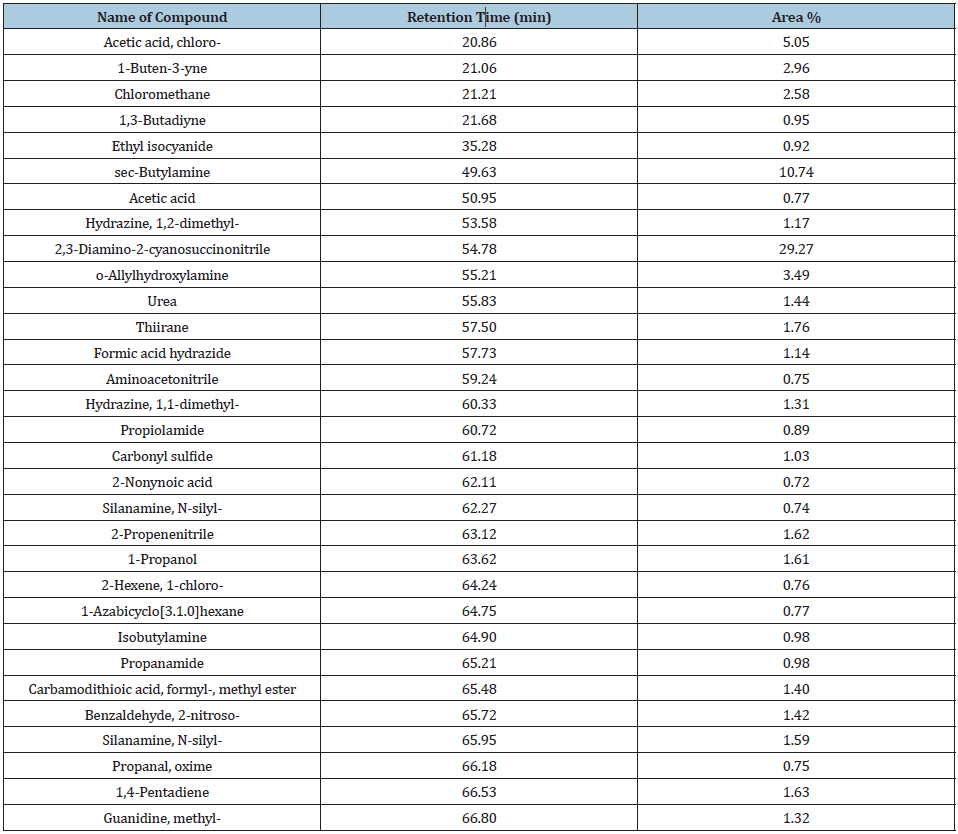
Some of the constituents detected in high amount include 2,3-Diamino-2-cyanosuccinonitrile, sec-Butylamine and Acetic acid, chloro-, while some of the constituents detected in low amount include Aminoacetonitrile, Propanal, oxime and 2-Nonynoic acid (Figure 1).
Figure 1:GCMS chromatogram of palm kernel oil
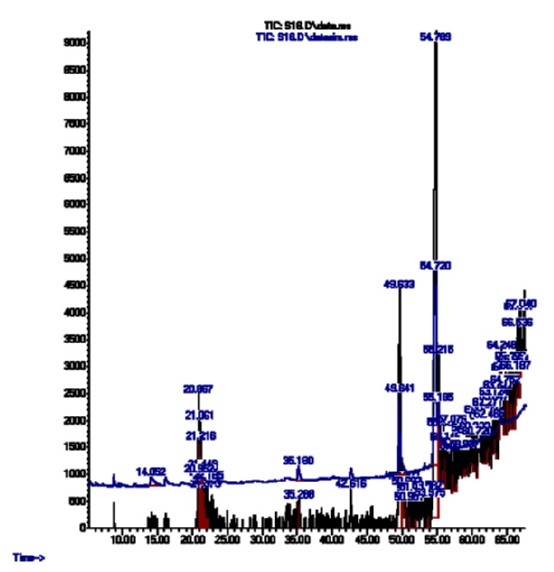
Various constituents identified in palm kernel oil (table 1 and figure 1) have been reported in literatures to exhibit different properties and functions as discussed below.
Chloroacetic acid has a role as an alkylating agent and a herbicide [7]. It has been reported to be used in the production of dyes, drugs and pesticides [8]. It has also been documented that exposure to concentrated chloroacetic acid or moderate air levels of chloroacetic acid may cause severe damage and burns to the skin, eyes, respiratory tract and mucous membranes. Cases of accidental exposure to concentrated chloroacetic acid directly to large areas of skin (>10% of body) was reported to cause coughing, disorientation, agitation, blood in the lungs, cardiac failure, general organ failure and death (Hazardous Substances Data Bank [9]). Large scale use of chloromethane is applied in the production of dimethyl-dichlorosilane and related organosilicon compounds. Inhalation of chloromethane gas produces central nervous system effects which is similar to alcohol intoxication, while its prolonged exposure may have mutagenic effects [10]. Another constituent detected in palm kernel oil known is 2-Propenenitrile, which is also known as Acrylonitrile is believed to be reactive and toxic at low doses, and to increase cancer in high dose. These functions of Chloroacetic acid, chloromethane and 2-Propenenitrile shows that high exposure of palm kernel oil may pose certain danger or toxic effects.
According to NCI Thesaurus a [11], 2-Propenenitrile (Acrylonitrile) is a colorless, volatile liquid with a pungent, onion-like odour. Acrylonitrile was stated to be widely used in industry to produce rubber, resins, plastics, elastomers and synthetic fibers. It is also used in manufacturing carbon fibers used in aircraft, defense and aerospace industries. Exposure to acrylonitrile may irritate the mucous membranes and causes headache, impaired judgment, nausea, dizziness, difficulty breathing, cyanosis, convulsions and collapse. Ethyl isocyanate is used for pesticide intermediate and pharmaceutical [12]. This implies that beside the negative effects, these constituents which can be extracted from palm kernel oil will possess these important industrial uses.
In a 2010 study, urea was reported to have been used to treat euvolemic hyponatremia and was also reported to be safe, inexpensive and simple [13]. Formic hydrazide was reported to be used in the production of 1,2,4-triazole derivatives [14] and of new anticancer agent: 6-N-formylamino-12,13-dihydro-1,11-dihydroxy-13-(β-D-glucopyranosyl)-5H-indolo[2,3-a] pyrrolo [3,4-c] carbazole-5,7(6H)-dione [15]. Acetic acid possesses antibacterial and antifungal properties. It also possesses the ability to inhibit carbohydrate metabolism resulting in subsequent death of the organism and used to dissolve substances required to make some medicines and other products such as plastics [16]. Aminoacetonitrile derivatives are useful antihelmintics. They act as nematode specific ACh agonists [17] causing a spastic paralysis and rapid expulsion from the host. These suggests palm kernel oil as a useful agent in some medical and pharmaceutical practices.
Various antimicrobial activities of some constituents of palm kernel oil has been reported. Sec-Butylamine has been reported to be a white liquid with an odour of ammonia and its contact with the eyes can cause lachrymation, conjunctivitis, burns and corneal edema, while its contact with the skin may cause irritation, burns and dermatitis [18]. In another research, Yoshikawa et al. [19] reported that sec-Butylamine at 5mM inhibited the oxidation of pyruvate by mitochondria isolated from hyphae of Penicillium digitatum. Sec-butylamine has been documented as a fumigant fungicide with a high potential for bioaccumulation, it is not approved for fungicidal use in the European Union, however, it has a role as an antifungal agrochemical. It is also documented as a primary aliphatic amine and an aliphatic nitrogen antifungal agent [20]. o-Allylhydroxylamine detected has been reported to possess antimicrobial activity against Plasmodium falciparum [21] and to be a useful intermediate; e.g. cyclization of o-allyloximes to give pyridines [22]. Thiiranes are known to be used as antimicrobial (fungicides) and also as insecticides. A thiirane derivative known as chlorocyclopropane sulfide is used as a nematocide [23]. This means that palm kernel oil will be used in the production of several antimicrobial agents.
Carbonyl sulfide which was also detected in the palm kernel oil has been reported as a potential alternative fumigant [24] to methyl bromide and phosphine. This implies that carbonyl sulfide and Sec-butylamine present in palm kernel oil may contribute to palm kernel oil been useful for the production of fumigants. The 1-propanol detected in palm kernel oil is known as a multi-purpose solvent in industry and in the home. It is used in some products such as cosmetics and lotions, polish and antiseptic formulations.
In nutrition, 2-Nonenoic acid is a food additive which is used as flavouring agents, while Isobutylamine has been reported in a research as an odourant binding to TAAR3 in mice and may trigger sexual behaviour in male mice dependent on the cluster of TAAR2 through TAAR9 [25]. Methylguanidine has been documented as a suspected uraemic toxin which accumulates in renal failure, but also exhibits anti-inflammatory effects. Methylguanidine is synthesized from creatinine concomitant with the production of hydrogen peroxide from endogenous substrates in peroxisomes. It is documented that evidence suggests that methylguanidine significantly inhibits iNOS activity and TNF- release. This implies that methylguandine may attenuate the degree of inflammation and tissue damage associated with endotoxic shock [26]. The implication of this is that palm kernel oil will possess anti-inflammatory properties. This may contribute to the reason why it is commonly used in traditional medicine.
Conclusion
The functions of some of the chemical constituents of palm kernel oil showed that palm kernel oil may be used for various purposes such as important antimicrobial agents, a useful agent in some medical and pharmaceutical practices and for industrial uses. It also contains chemical constituents that shows that high exposure of palm kernel oil may pose certain danger or toxic effects.
References
- Ugbogu OC, Onyeagba RA, Chigbu OA (2006) Lauric acid content and inhibitory effect of palm kernel oil on two bacterial isolates and Candida albicans. African Journal of Biotechnology 5: 1045-1047.
- Imo C, Sunday OD (2020) Comparative Effects of Palm Kernel Oil, Olive Oil, Crude Oil and Honey on Lipid Profile, Body Weight and Hearts of Male Albino Rats. European Journal of Biomedical and Pharmaceutical Sciences 7(5): 84-90.
- Bredeson DK (1983) Mechanical Oil Extraction. Journal of the American Oil Chemists' Society 60(2): 211-213.
- Imo C, Arowora KA, Abu MS, Angbas FA (2020) Comparative Effects of Palm Kernel Oil, Olive Oil, Crude Oil and Honey on Liver Function of Male Albino Rats. European Journal of Pharmaceutical and Medical Research 7(5): 26-31.
- Sutapa M, Analava M (2009) Health effects of Palm Oil. Journal of Humidity and Ecology 26(3): 197–203.
- Imo C, Yakubu OE, Imo NG, Udegbunam IS, Onukwugha OJ (2018) Chemical composition of Xylopia aethiopica American Journal of Physiology, Biochemistry and Pharmacology 7: 48–53.
- Chemical Entities of Biological Interest a (ChEBI a).
- Koenig G, Lohmar E, Rupprich N (2005) Chloroacetic Acids. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim, Germany.
- Hazardous Substances Data Bank (HSDB).
- Rossberg M, Lendle W, Pfleiderer G, Tögel A, Dreher EL, et al. (2006) Chlorinated Hydrocarbons. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim, Germany.
- NCI Thesaurus a (NCIt a).
- Lewis RJ (2001) Hawley's Condensed Chemical Dictionary 14th John Wiley & Sons, Inc. New York, USA, pp. 472, 738, 927.
- Decaux G, Andres C, Gankam KF, Soupart A (2010) Treatment of euvolemic hyponatremia in the intensive care unit by urea. Crit Care14(5): R184.
- Zong Y, Wang J, Yue G, Feng L, Song Z, et al. (2005) Traceless liquid-phase synthesis of 3-alkylamino-4,5-disubstituted-1,2,4-triazoles on polyethylene glycol (PEG). Tetrahedron Letters 46(31): 5139-5141.
- Ohkubo M, Kawamoto H, Ohno T, Nakano M, Morishima H (1997) Synthesis of NB-506, a new anticancer agent. Tetrahedron 53(2): 585-592.
- NCI Thesaurus b.
- Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, et al. (2008) A new class of anthelmintics effective against drug-resistant nematodes. Nature 452(7184): 176-180.
- US Coast Guard (1999) Chemical Hazard Response Information System (CHRIS) - Hazardous Chemical Data. Commandant Instruction 16465.12C. Government Printing Office, Washington DC, USA.
- Yoshikawa M, Eckert JW, Keen NT (1976) The mechanism of fungistatic action of sec-butylamine: II. The effect of sec-butylamine on pyruvate oxidation by mitochondria of Penicillium digitatum and on the pyruvate dehydrogenase complex. Pestic Biochem Physiol 6(5): 482-490.
- Chemical Entities of Biological Interest b (ChEBI b).
- Prado Prado FJ, García Mera X, González Díaz H (2010) Multi-target spectral moment QSAR versus ANN for antiparasitic drugs against different parasite species. Bioorg Med Chem 18(6): 2225-2231.
- Kusumi T, Yoneda K, Kakisawa H (1979) A Convenient Synthesis of 5,6,7,8-Tetrahydroquinoline. Synthesis 221-221.
- Vishnu JR, Arun S, Mahendra N, Ramendra P (2019) Three-Membered Ring Heterocycles. In The Chemistry of Heterocycles pp: 19-92.
- Bartholomaeus A, Haritos V (2005) Review of the toxicology of carbonyl sulfide, a new grain fumigant. Food Chem Toxicol 43(12): 1687–1701.
- Harmeier A, Meyer CA, Staempfli A, Casagrande F, Petrinovic MM, et al. (2018) How Female Mice Attract Males: A Urinary Volatile Amine Activates a Trace Amine-Associated Receptor That Induces Male Sexual Interest. Front Pharmacol 9: 924.
- Human Metabolome Database (HMDB).
© 2021 Chinedu Imo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






