- Submissions

Full Text
Advances in Complementary & Alternative medicine
Evaluation of Vitamin D Receptor (VDR) Expression By RT-PCR Method Using Biofield Energy Treated Proprietary Test Formulation in Six Different Cell Lines
Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1 and Snehasis Jana2*
1Trivedi Global, Inc., Henderson, Nevada, USA
2Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
*Corresponding author:Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
Submission: July 10, 2021;Published: July 26, 2021

ISSN: 2637-7802 Volume 6 Issue 4
Abstract
The aim of the present study was to evaluate the vitamin D receptor (VDR) expression after treatment with the Trivedi Effect®- Biofield Energy Treated (Blessed) Test formulation/item (TI). The novel test formulation was composed of minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, alpha tocopherol, cyanocobalamin, and cholecalciferol), Panax ginseng extract, CBD isolates, and β-carotene, which was tested on various cell lines in DMEM medium using values of relative quantification (RQ) by RT-PCR among different groups. The test formulation constituents were divided into two parts; untreated test formulation (UT), another part received Biofield Energy Healing Treatment (BT) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. MTT data showed that all the cell lines after treatment with the test formulation in various tested concentrations were found as safe and nontoxic with cell viability ranges from 71% to 181% upto 10 μg/mL. A549 cells showed a significant increased RQ values by 29.9% (UT- DMEM + BT-TI) and 200.2% (BT-DMEM + UT-TI) among the experimental groups as compared with the untreated test formulation. HepG2 cells showed a significant increased RQ value by 10.3% to 233.1% in the UT- DMEM + BT-TI group at 0.01 and 1μg/mL, respectively as compared with the untreated test group. HT-29 cells showed a significant increased RQ value by 34.8% (BT-DMEM + UT-TI group) to 220% (BT-DMEM + BT-TI group); while EA.hy926 cells significantly increased RQ value by 20.9% (BT-DMEM + BT-TI) to 398.8% (UT- DMEM + BT-TI) among the tested group as compared with the untreated test group. C2C12 cells showed a significant increased RQ value by 6.3% to 100% in the BT-DMEM + BT-TI group as compared with the untreated test group. Hence, it can be concluded that the Biofield Energy Treatment (Blessing) may have the potential to upregulate the VDR expression in multiple cell-lines. Overall, the results showed the significant increased VDR expression in terms of increase RQ values in all the tested cell-lines. Hence, it can be concluded that the Biofield Energy Treatment (Blessing) may have the excellent potential to upregulate the VDR expression in multiple cell-lines, and that could help calcium and bone homeostasis, and slowdown of the disease progression rate related to vitamin D3 deficiency.
Keywords: Biofield energy; MTT assay; The Trivedi effect®; VDR expression
Introduction
From last two decades, immense research have been performed with respect to various biological actions of 1, 25-dihydroyxyvitamin D3 (1, 25(OH)2D3) via precise changes in gene expression through regulation of intracellular vitamin D receptor (VDR) [1]. VDR activation is via direct interaction with 1,25(OH)2D3 which regulates the receptor’s rapid binding capacity for respective target genes, which results in the formation of large protein complexes that are responsible for many biological activities [2]. Calcitriol regulates its function by mediating using ligand-inducible transcription factor (VDR). Further, it is associated with calcium and bone homeostasis along with other cellular processes, such as proliferation and differentiation. VDR expression depends on the binding with specific DNA elements of target genes and inducing or repressing various transcriptional activities [3,4]. VDR expression
is regulated by many factors such as hormones, growth factors, and
calcium [5-7]. There are certain factors that may improve the VDR
expression (cAMP-activated protein kinase A), while other (protein
kinase C) may reduced [8]. A549 (Human Lung adenocarcinoma),
HepG2 (Human Hepatocarcinoma Cells), HT-29 (Human Colorectal
adenocarcinoma), EA.hy926 (Human Endothelial cells), THP-1
(Human Leukemia Cells) and C2C12 (Mouse Myoblast Cells) cell line
were used in order to detect the expression of VDR after treatment
with the Biofield Energy Healing-based novel test formulation. The
novel test formulation was composed of minerals (Ca, Zn, Mg, Se,
Fe, Cu), vitamins (B12, E, D3, C, B6), and some biological active plantbased
extracts such as β-carotene, Ginseng, and cannabidiol isolate
(CBD). Vitamins and minerals used in the novel formulation have
reported with significant biological activity [9-11]. Cannabidiol
used in the formulation is reported with wide range of biological
action [12,13], while ginseng extract is one of the best immune
booster for overall biological activity [14]. Vitamins are associated
with immunity builder and works through various pathways for
bone health. Vitamin D is reported for improved strength, skin
elasticity [15,16], improve arterial stiffness, neuronal plasticity, and
many more [17-19].
Biofield Energy Healing Therapy is believed to flow throughout
the human body, and it can be measured to some extend via
conventional instrumentation. Complementary and Alternative
Medicines (CAMs) are one of the major sections of medicine, which
was reported with significant results in many scientific disciplines,
which are increasingly popular in the developing and developed
countries [20]. Biofield Energy Healing therapy is acceptance
worldwide and National Center for Complementary and Alternative
Medicine (NCCAM) has been inaugurated US research agencies
for conducting scientific research and practicing in the arena of
medicine. Biofield Energy Therapy is highly responsive with respect
to the physical, mental, and emotional human wellness [20], which
improved the endogenous energy flows. In addition, CAM therapies
have been accepted by the National Institute of Health (NIH) and
National Centre of Complementary and Integrative Health (NCCIH)
along with the Biofield Energy Healing, such as deep breathing,
Tai Chi, yoga, therapeutic touch, Reiki, chiropractic/osteopathic
manipulation, relaxation techniques, pranic healing, meditation,
homeopathy, Ayurvedic medicine, movement therapy, mindfulness,
traditional Chinese herbs and medicines in biological systems,
etc. [21]. However, Trivedi Effect®-Consciousness Energy Healing
Treatment have significant clinical, preclinical, and scientific
studies in different scientific disciplines such in agriculture science
[22], materials science [23,24], bioavailability studies [25,26],
microbiology [27,28], skin health [29,30], bone health [31,32],
biotechnology [33], herbomineral products [34], cancer research
[35], and overall human health and wellness. The present study
demonstrated the effect of Biofield Energy Treatment on media
(DMEM) and a novel test formulation for its VDR expression using
A549, HepG2, HT-29, EA.hy926, THP-1, and C2C12 cell lines through
the values of relative quantification (RQ) in RT-PCR.
Material and Methods
Chemicals and cell-lines
Pyridoxine hydrochloride (vitamin B6), calcitriol, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, provit A) were purchased from TCI, Japan. Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (alpha-tocopherol), cholecalciferol (vitamin D3), iron (II) sulfate, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma- Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Cannabidiol isolate and Panax ginseng extract were obtained from Standard Hemp Company, USA, and Panacea Phytoextracts, India, respectively. Resveratrol was purchased from Acros Organics, and DMEM was purchased from Lonza, USA. A549 (Human Lung adenocarcinoma), HepG2 (Human Hepatocarcinoma Cells), HT-29 (Human Colorectal adenocarcinoma), EA.hy926 (Human Endothelial cells), THP-1 (Human Leukemia Cells) and C2C12 (Mouse Myoblast Cells) cell line were procured from ATCC, USA.
Cell culture
All the cell lines were used as test system, which were maintained in specific growth medium such as DMEM for routine culture supplemented with 10% FBS. Growth conditions were maintained at 37 °C, 5%CO2, and sub-cultured by trypsinization followed by splitting the cell suspension into fresh flasks and supplementing with fresh cell growth medium. Three days before the start of the experiment (i.e., day -3), the growth medium of near-confluent cells was replaced with fresh phenol-free medium, supplemented with 10% charcoal-dextran stripped FBS (CD-FBS) and 1% penicillin-streptomycin.
Experimental design
The experimental groups consisted of cells in baseline control, vehicle control groups (0.05% DMSO with Biofield Energy Treated and untreated DMEM), positive control group (resveratrol) and four different experimental test groups. The experimental groups included the combination of the Biofield Energy Treated and untreated test formulation/Medium (DMEM). It consisted of four major treatment groups on specified cells with Untreated (UT)- DMEM + UT-Test item (UT-TI), UT- DMEM + Biofield Energy Treated test item (BT-TI), BT- DMEM + UT-TI, and BT- DMEM + BT-TI.
Consciousness energy healing strategies
The novel test formulation was divided into two parts. One part of the test compound did not receive any sort of treatment and was defined as the untreated test formulation. The second part of the test formulation was treated with the Trivedi Effect® - Energy of Consciousness Healing Treatment/Blessing (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for about 3 minutes through Trivedi’s unique Energy Transmission process. The Blessing/Treatment was given to the test formulation without touching in the laboratory of Dabur Research Foundation, near New Delhi, India. After that, the Biofield Energy Treated/Blessed samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer for 3 minutes, under the same laboratory conditions. The “sham” healer did not have any knowledge about the Biofield Energy Treatment/Blessing. The Biofield Energy Treated/Blessed test medium was also taken back to experimental room for further culture methods.
Determination of non-cytotoxic concentration
All the cells were used in the assay with cellular density 10000 cells/ well contained 25000 cells/ well. The single cell suspension of all the cells were prepared with 10% FBS. The cells were counted on a hemocytometer, while the cells were seeded with specific cell density in 96-well plates. The cells were incubated in a CO2 incubator for 24 hours. After 24 hours, medium was removed, and following treatments were given in medium along with the 10% FBS in various experimental groups. After incubation for 24 hours, the effect of the test formulation on cell viability was assessed by 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. About 20μL of 5mg/mL of MTT was added to all the wells and incubated at 37 °C for 3 hours. The cells were centrifuged to obtain the pellet. The supernatant was removed and 150μL of DMSO was added to all wells to dissolve formazan crystals. Further, all the wells were reported using optical density (OD) values at 540nm using Synergy HT microplate reader. The effect of the test formulation on viability of cells was determined using equation 1.
%Cell viability = 100 - % Cytotoxicity--------------------- (1)
Where, %Cytotoxicity = {(O.D. of Control cells – O.D. of cells treated with test formulation)/ OD of Control cells} *100
Estimation of VDR expression
The single cell suspension of all the cells was prepared in 10% FBS along with its specific medium. The cells were counted using a hemocytometer and plated in 35mm culture dishes. The cells were incubated overnight under growth conditions to allow cell recovery and exponential growth. Following overnight incubation, the above cells were subjected to serum starvation in DMEM + 10% FBS. The cells were then treated with different concentrations of test formulation obtained by serial dilution of main stock (i.e., 19.94mg/ mL in DMSO stock) in DMEM. Following respective treatments, each set was incubated in a CO2 incubator at 37 °C, 5% CO2, and 95% humidity and incubated for 24 hours. The cells were harvested by scrapping and washed with PBS. The cell pellets obtained were analyzed for VDR gene expression using human VDR specific primers such as forward: 5’-GCTGACCTGGTCAGTTACAGCA-3’, and reverse: 5’-CACGTCACTGACGCGGTACTT-3’. The VDR gene expression was normalized using Internal Control (IC) reference. Relative quantification (RQ) of VDR gene in treated cells was calculated with respect to the untreated cells using following formula:
Where, N is the relative Threshold Cycle (CT) value of treated sample with respect to the untreated sample.
Statistical analysis
The data were represented as mean±standard error of mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison Oneway analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 was considered as statistically significant.
Results
MTT assay- non-cytotoxic effect of the test formulation
The cytotoxic effect of the test formulation was evaluated on A549 (Human Lung adenocarcinoma), HepG2 (Human Hepatocarcinoma Cells), HT-29 (Human Colorectal adenocarcinoma), EA.hy926 (Human Endothelial cells), THP-1 (Human Leukemia Cells), and C2C12 (Mouse Myoblast Cells) cells using MTT assay. The cells were treated with the test formulation for 24 hours. The effect on viability of cells was determined after 24 hours of treatment by MTT assay. The cells were treated with the test formulation and in various experimental test groups. Calcitriol upto 1000nM in the A549 cell line showed upto 112% cell viability, while in experimental test groups showed cell viability range from 75% to 116% upto 10 μg/ mL test concentration. Similarly, the HepG2 cell line showed cell viability upto 122% in positive control (calcitriol), while upto 105% in experimental test group with upto 25μg/mL test formulation concentration. Calcitriol at 100, 500, and 1000nM in HT-29 cell line showed upto 126.6% cell viability, while in experimental test groups showed cell viability range from 84% to 181% upto 25μg/ mL test concentration. Cell viability in EA.hy926 cells showed upto 125% in calcitriol group, while cell viability range is from 91% to 131% in experimental test group with concentration upto 5μg/mL. THP-1 cell line showed upto 109% cell viability in calcitriol group upto 500nM, while experimental test groups showed cell viability range from 71% to 99% upto 10μg/mL test concentration. C2C12 cell line showed upto 110% cell viability in calcitriol group upto 500 nM, while experimental test groups showed cell viability range from 99% to 166% upto 25μg/mL test concentration. Overall, the MTT data of all the tested cell lines suggested that the test formulation along with test media groups were found safe at all the tested concentrations range up to maximum 10 to 25μg/mL in the respective cell lines.
Assessment of the test formulation on VDR expression
The VDR expression in all the cells were counted using a hemocytometer and plated in 35mm culture dishes. The cells were treated with the test formulation at different combinations and effect on VDR expression was determined using quantitative- PCR amplification. All the cell lines showed significant improved RQ values with respect to untreated test formulation group except THP-1 cell line. VDR-CT values were obtained from PCR amplification. Relative quantification (RQ) was calculated from the VDR-CT and IC-CT values for the different cell lines treated with the test formulation.
VDR expression of A549 cell line
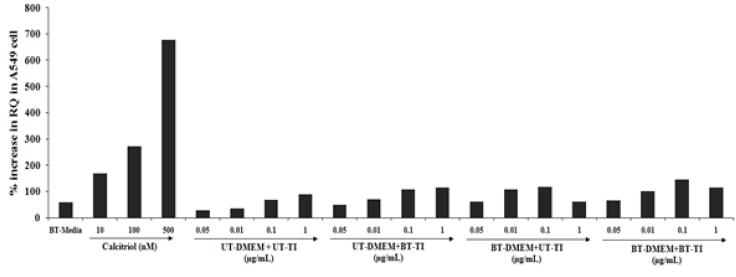
The effect of the test formulation on VDR expression in the A549 cells showed a significant increased RQ values in all the test formulation concentration combination groups. The positive control, calcitriol showed 169.4%, 273.2%, and 678.1% increased RQ value as compared with the normal control group at 10, 100, and 500nM concentrations, respectively. However, the experimental treated groups such as UT- DMEM + Biofield Energy Treated test item (BT-TI) group showed improved value of RQ by 94.5%, 61.9%, and 29.9% at 0.05, 0.01, and 0.1μg/mL, respectively as compared with the untreated test formulation, which suggest VDR expression was significantly modulated. Similarly, 200.2%, 75.1%, and 115.3% increased RQ value at 0.05, 0.01, and 1μg/mL, respectively as compared with the untreated test formulation in the BT-DMEM + UT-TI group. However, BT-DMEM + BT-TI group showed improved RQ value by 184.3%, 115.7%, 28.2%, and 99.4% at 0.05, 0.01, 0.1, and 1μg/mL, respectively as compared with the untreated test formulation group (Figure 1).
Figure 1:The effect of the test formulation on VDR expression in A-549 cell line. UT: Untreated; BT: Biofield Treated; TI: Test Item.
VDR expression of HepG2 cell line
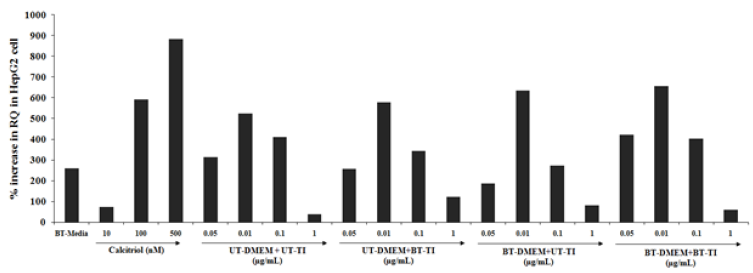
The HepG2 cells VDR expression showed a significant increased RQ value in all the test formulation groups. The positive control, calcitriol showed 74.1%, 591.6%, and 884.9% increased value of RQ as compared with the normal control group at 10, 100, and 500nM concentrations, respectively. However, the experimental treated groups such as UT- DMEM + Biofield Energy Treated test item (BTTI) group showed improved value of RQ by 10.3% and 233.1% at 0.01 and 1μg/mL respectively, as compared with the untreated test formulation. Similarly, 21.6% and 122.7% increased RQ value at 0.01 and 1μg/mL, respectively as compared with the untreated test formulation in the BT-DMEM + UT-TI group. However, the BT-DMEM + BT-TI group showed an improved RQ value by 33.9%, 25.5%, and 61.5% at 0.05, 0.1, and 1μg/mL, respectively as compared with the untreated test formulation (Figure 2).
Figure 2:The effect of the test formulation on VDR expression in HepG2 cell line. UT: Untreated; BT: Biofield Treated; TI: Test Item.
VDR expression of HT-29 cell line
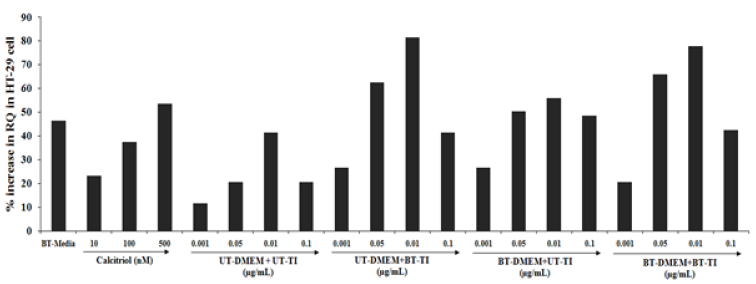
The HT-29 cells were used for the estimation of VDR expression. The positive control, calcitriol showed 23.1%, 37.6%, and 53.7% increased value of RQ as compared with the normal control group at 10, 100, and 500nM concentrations, respectively. Similarly, the experimental treated groups such as UT- DMEM + Biofield Energy Treated test item (BT-TI) group showed improved value of RQ by 126.6%, 203.4%, 96.8%, and 101.3% at 0.001, 0.01, 0.05, and 0.1μg/mL, respectively as compared with the untreated test formulation. Similarly, BT-DMEM + UT-TI group showed improved RQ value by 126.6%, 145.5%, 34.8%, and 135.4% at 0.001, 0.05, 0.01, and 0.1μg/mL respectively, as compared with the untreated test formulation. However, the BT-DMEM + BT-TI group showed an improved RQ value by 75.5%, 220%, 87.7%, and 106% at 0.001, 0.05, 0.01, and 0.1μg/mL, respectively as compared with the untreated test formulation group (Figure 3).
Figure 3:The effect of the test formulation on VDR expression in HT-29 cell line. UT: Untreated; BT: Biofield Treated; TI: Test Item.
VDR expression of EA.hy926 cell line
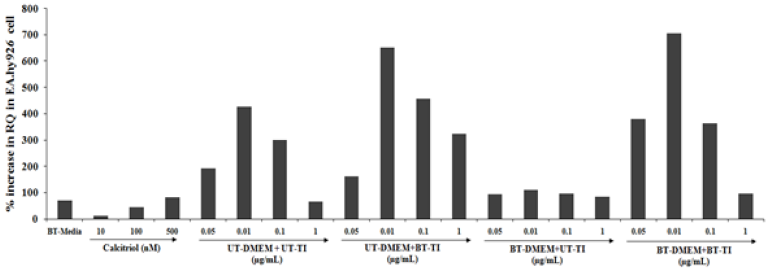
The EA.hy926 cells also showed significant results with respect to the VDR expression with increased RQ value in all the test formulation groups. The positive control, calcitriol showed 11.7%, 44.4%, and 81.5% increased value of RQ as compared with the normal control group at 10, 100, and 500nM concentrations, respectively. Similarly, the experimental treated groups such as UTDMEM + Biofield Energy Treated test item (BT-TI) group showed improved value of RQ by 52.3%, 52.6%, and 398.8% at 0.001, 0.05, 0.01, and 0.1μg/mL respectively as compared with the untreated test formulation. Similarly, BT-DMEM + UT-TI group showed improved RQ value by 31.8% at 1μg/mL respectively, as compared with the untreated test formulation. However, the BT-DMEM + BTTI group showed an improved RQ value by 96.6%, 64.9%, 20.9%, and 48.2% at 0.05, 0.01, 0.1, and 1μg/mL respectively, as compared with the untreated test formulation (Figure 4).
Figure 4:The effect of the test formulation on VDR expression in EA.hy926 cell line. UT: Untreated; BT: Biofield Treated; TI: Test Item.
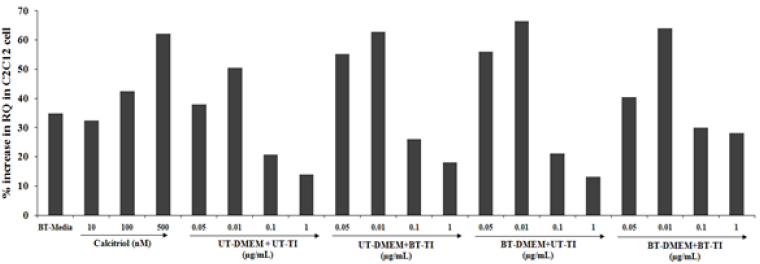
VDR expression of C2C12 cell line
The C2C12 cells also showed significant results with respect to the VDR expression with increased RQ value in all the test formulation groups. The positive control, calcitriol showed 32.5%, 42.5%, and 62.1% increased value of RQ as compared with the normal control group at 10, 100, and 500 nM concentrations, respectively. Similarly, the experimental treated groups such as UT- DMEM + Biofield Energy Treated test item (BT-TI) group showed improved value of RQ by 45.3%, 24.6%, 25%, and 28.6% at 0.05, 0.01, 0.1, and 1μg/mL respectively as compared with the untreated test formulation. Similarly, BT-DMEM + UT-TI group showed improved RQ value by 47.4%, 31.7%, and 1.9% at 0.05, 0.01, and 0.1μg/mL respectively, as compared with the untreated test formulation. However, the BT-DMEM + BT-TI group showed an improved RQ value by 6.3%, 27%, 44.2%, and 100% at 0.05, 0.01, 0.1, and 1μg/mL respectively, as compared with the untreated test formulation (Figure 5).
Figure 5:The effect of the test formulation on VDR expression in C2C12 cell line. UT: Untreated; BT: Biofield Treated; TI: Test Item.
Discussion
Vitamin D, is considered to show pleiotropic effect along
with its traditional role in calcium homeostasis. Cell cycling,
proliferation, differentiation, and apoptosis is controlled by many
genes along with VDR elements. VDR elements (VDREs) are related
with many genes, which may include a multitude of fundamental
cellular processes or VDR expression in many cell lines [36,37].
Vitamin D, its active metabolite 1,25-dihydroxyvitamin D
(calcitriol) and other compounds have been reported in the cellspecific
manners, that regulates the cellular proliferation process
via multiple mechanisms. The most preferably mechanism is via
effects on cell cycle progression, apoptosis, and differentiation [38].
Calcitriol regulates calcium homeostasis, immunity, and cellular
growth and differentiation [15]. Calcitriol inhibit the proliferation
and/or to induce the differentiation of various types of malignant
cells, including breast, prostate, colon, skin, and brain cancer cells,
as well as myeloid leukemia cells in vitro [39]. It mediates via
VDR that regulates the rates of transcription of key target genes
involved directly or indirectly in calcium and phosphate regulation
[40]. VDR has been reported in multiple tissues and cell types
like skin, muscle, breast, colon and hematopoietic cells [41]. VDR
application was reported in hyper-proliferative skin diseases,
hyperparathyroidism, osteoporosis, cancer, CVD, infections, and
autoimmune diseases [42].
According to Medeiros JFP et al., reported that supplementation
with cholecalciferol (vitamin D3) showed an increase serum
concentration of vitamin D3 and VDR gene expression sixty times.
Also reported with changes in body composition parameters like
body fat and lean mass [43]. Additionally, most of biological actions
of vitamin D exerted through VDR-mediated control of target genes
[44]. Thus, in this experiment a gene expression study of vitamin
D one of the ingredient supplementation of tested proprietary test
formulation is an innovative idea. In the present study, the effect
of the Biofield Energy Treatment/Blessing on VDR expression was
determined in different cell lines of different origin. The results
showed the significant VDR expression in all the tested cell lines,
which helps in slowdown of the disease progression rate related
to vitamin D3 deficiency, stress-related all other symptoms/
complications and also reduced the chances of disease susceptibility.
This improved cellular differentiation, contractile functions, exonal
extensions, and skin elasticity and firmness of the cell lines used
in the study after treatment was very significant. Based on the
overall data, it suggests that the Biofield Energy Healing Therapy
was found to be most effective and benefited in order to prevent
and protect from the occurrence of any type of diseases and can
be used as significant way for energy boosting in various disease
states that will ultimately improve the overall health and quality of
life in human.
Conclusion
The VDR expression was evaluated using different cell lines such as A549, HepG2, HT-29, EA.hy926, THP-1, and C2C12. Further, MTT assay were tested and the effect of test formulation was evaluated at different concentrations that was compared with respect to the positive control and untreated test formulation. MTT analysis showed that all the test formulation concentrations and combinations are found to be non-toxic with viability range 71% to 181% among the all five tested cell lines up to maximum 10μg/ mL test formulation concentration. After confirming the safety profile using MTT in all the cell lines, relative quantification (RQ) was calculated from the VDR-CT and IC-CT values using RT-PCR. RQ values in A549 cell line showed significant increased values by 29.9% to 200.2% among the experimental groups as compared with the untreated test formulation group. Similarly, HepG2 cells showed a significant increased RQ value in all the test formulation groups by 10.3% to 233.1% among the tested groups as compared with the untreated test group. HT-29 cells were used for the VDR expression in the showed a significant increased RQ value by 34.8% to 220%, while EA.hy926 cells significant increased RQ value by 20.9% to 398.8% among the tested groups as compared with the untreated test group. C2C12 cells showed a significant increased RQ value by 1.9% to 100% among the tested groups as compared with the untreated test group. Thus, it can be concluded that the Biofield Energy Treatment may have the potential to modulate Vitamin D3 metabolism via VDR expression. Overall, the Biofield Energy Treated (the Trivedi Effect®) test formulation showed a significant improved VDR expression in the tested cell lines, which play a vital role in maintaining various immune and life style-related disorders such as Alzheimer’s, cardiovascular, cancer, diabetes, Parkinson’s. Therefore, the Consciousness Energy Healing-based test formulation might be suitable an alternative nutritional supplement, which could be useful for the management of various immune-related disorders.
Acknowledgement
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
References
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, et al. (1998) The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res 13(3): 325-349.
- Sutton AL, MacDonald PN (2003) Vitamin D: More than a “bone-a-fide” hormone. Mol Endocrinol 17(5): 777-791.
- Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81(3): 1269-1304.
- Dowd DR, Sutton AL, Zhang C, MacDonald P (2005) Comodulators of VDR-mediated gene expression. In: Feldman D, Pike JW, Glorieux FH, (Eds.) Vitamin D. Elsevier Academic, Burlington, Massachusetts, USA, Pp. 291-304.
- Petkovich PM, Heersche JN, Tinker DO, Jones G (1984) Retinoic acid stimulates 1,25-dihydroxyvitamin D3 binding in rat osteosarcoma cells. J Biol Chem 259(13): 8274-8280.
- Walters MR (1981) An estrogen-stimulated 1,25-dihydroxyvitamin D3 receptor in rat uterus. Biochem Biophys Res Commun 103(2): 721-726.
- Uhland-Smith A, DeLuca HF (1993) The necessity for calcium for increased renal vitamin D receptor in response to 1,25-dihydroxyvitamin D. Biochem Biophys Acta 1176(3): 321-326.
- Krishnan AV, Feldman D (1991) Activation of protein kinase-C inhibits vitamin D receptor gene expression. Mol Endocrinol 5(4): 605-612.
- Byrne JH, Voogt M, Turner KM, Eyles DW, McGrath JJ, et al. (2013) The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague-Dawley rats. PLoS One 8(8): e71593.
- Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225): 233-241.
- Beard JL, Connor JR (2003) Iron status and neural functioning. Ann Rev Nutr 23: 41-58.
- Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, et al. (2018) Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol 9: 482.
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1(7): 1333-1349.
- Kang S, Min H (2012) Ginseng, the 'immunity boost': The effects of Panax ginseng on immune system. J Ginseng Res 36(4): 354-368.
- Schagen SK, Zampeli VA, Makrantonaki E, Zouboulis CC (2012) Discovering the link between nutrition and skin aging. Dermatoendocrinol 4(3): 298-307.
- Mostafa WZ, Hegazy RA (2015) Vitamin D and the skin: Focus on a complex relationship: A review. J Adv Res 6(6): 793-804.
- Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, et al. (2010) A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab 95(10): 4584-4591.
- Hwang E, Park SY, Yin CS, Kim HT, Kim YM, et al. (2017) Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J Ginseng Res 41(1): 69-77.
- https://www.redstormscientific.com/top-15-health-benefits-of-cbd-oil-cannabidiol/
- Maizes V, Rakel D, Niemiec C (2009) Integrative medicine and patient-centered care. Explore (NY) 5(5): 277-289.
- Bischof M, Del Giudice E (2013) Communication and the emergence of collective behavior in living organisms: A quantum approach. Mol Biol Int 2013: 987549.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica ). Journal of Food and Nutrition Sciences 3(6): 245-250.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Met Powder Rep 63(9): 22-28, 31.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4(3): 161.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague dawley International Journal of Clinical and Developmental Anatomy 3(3): 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague dawley American Journal of Clinical and Experimental Medicine 5(4): 138-144.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3(1): 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. J Antivir Antiretrovir 7(3): 083-088.
- Kinney JP, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. American Journal of Life Sciences 5(2): 65-74.
- Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. American Journal of Pharmacology and Phytotherapy 2(1): 1-10.
- Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. International Journal of Biomedical Engineering and Clinical Science 4(1): 6-14.
- Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). International Journal of Nutrition and Food Sciences 7(1): 30-38.
- Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ Health Sci 1: 1-9.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. International Journal of Bioorganic Chemistry 2(3): 135-145.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4(3): 141.
- Dusso AS, Brown AJ, Slatopolsky E (2005) Vitamin D. Am J Physiol Renal Physiol 289(1): F8-F28.
- Nagpal S, Na S, Rathnachalam R (2005) Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26(5): 662-687.
- Banerjee P, Chatterjee M (2003) Antiproliferative role of vitamin D and its analogs – a brief overview. Mol Cell Biochem 253(1-2): 247-254.
- Pirotta S, Kidgell DJ, Daly RM (2015) Effects of vitamin D supplementation on neuroplasticity in older adults: A double-blinded, placebo-controlled randomised trial. Osteoporos Int 26(1): 131-140.
- Daly R (2013) Oral Poster Presentations: Clinical #FR0195. the American Society for Bone and Mineral Research 2013 Annual Meeting, Baltimore, USA.
- Kogan NM, Mechoulam R (2007) Cannabinoids in health and disease. Dialogues Clin Neurosci 9(4): 413‐
- Wee JJ, Mee Park K, Chung AS (2011) Biological Activities of Ginseng and Its Application to Human Health. In: Benzie IFF, Wachtel-Galor S (Eds.) Herbal Medicine: Biomolecular and Clinical Aspects. 2nd Chapter 8, CRC Press/Taylor & Francis, Boca Raton, Florida, USA.
- Medeiros JFP, de Oliveira Borges MV, Soares AA, JC dos Santos, Ana BB de Oliveira et al. (2020) The impact of vitamin D supplementation on VDR gene expression and body composition in monozygotic twins: Randomized controlled trial. Sci Rep 10: 11943.
- Kato S (2000) The function of vitamin D receptor in vitamin D action. J Biochem 127(5): 717-722.
© 2021 Snehasis Jana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






