- Submissions

Full Text
Advances in Complementary & Alternative medicine
Histopathological Analysis of Cerebellum Layers in Rats Treated With Extract of Trichosanthes cucumerina: Neuroprotective Functions
Utibe Udonwa1*, Olaitan R Asuquo1, Marvin Okon2 and Thomas I Nathaniel3
1Department of Anatomy, Faculty of Basic Medical Sciences, University of Calabar, Nigeria
2Department of Medicine, Faculty of Health Sciences, Obafemi Awolowow University Ile-Ife,Nigeria
3University of South Carolina School of Medicine Greenville, USA
*Corresponding author: Utibe Udonwa, Department of Anatomy, Faculty of Basic Medical Sciences, University of Calabar, Nigeria
Submission: March 13, 2020;Published: April 07, 2020

ISSN: 2637-7802 Volume 6 Issue 2
Abstract
Herbal medicine provides neuroprotective functions for different brain disorders, and Trichosanthes cucumerina leaf extracts may provide neuroprotective function on the cerebellum. Evaluation of the neuroprotective potential of Trichosanthes cucumerina (TC) on the cerebellum is carried out. A total of thirty-two (32) adult rats with an average weight of 200g are being used to determine the effect of methanolic extract of Trichosanthes cucumerina leaf on the cerebellum for a period of twenty-eight (28) days. The rats are divided into 4 groups (A-D) with eight rats in each group. Group A animals receive feed and distilled water and serve as control. The animals in group B, C and group D receive 200mg/kgBW, 400mg/kgBW and 600mg/kgBW of methanolic extract of Trichosanthes cucumerina leaf respectively. 24hours after the last administration of the leaf extract, the animals are sacrificed using chloroform inhalation method. The cerebellum is harvested and fixed in 10% formalin. After fixation, the animals are passed through several stages of tissue processing which comprises dehydration, wax impregnation, embedding and sectioning. The sections are taken at 5μ and haematoxylin and eosin staining method is employed for the histological demonstration of the cerebellum. Microscopy and histochemical data of methanolic extract of T. cucumerina leaf show no adverse histopathological change in cerebellum of all treated groups of rats as they retain their normal cytoarchitecture when compared to the control group. The presence of biologically active phytochemicals including Cucurbitacin in Trichosanthes cucumerina may provide neuroprotective functions especially since the dosage given in the different treatments probably crossed the blood brain barrier and did not produce any pathological effect.
Keywords: Cerebellum; Histopathology; Neuroprotection; Trichosanthes cucumerina; Rats
Introduction
The need for man to survive has led him to discover many plants that are of medicinal value even before the advent of orthodox medicine. Plant-based medicines have been man’s prime therapeutic weapons from ancient times and are still in the uttermost significant form of treating diverse pathological conditions [1]. Herbal medicine involves the use of whole plants or part of plants such as seeds, berries, leaves, roots, barks and flowers to prevent or treat illness [2]. Herbal medicine has been reported safe and without any adverse effect especially when compared with synthetic drugs [3]. Therefore, a search for new drugs with better and cheaper substitutes from plant origin is a natural choice [4]. Herbal medicine requires testing for its efficacy and effectiveness since they do carry minor risks [5] but they are regarded by the public and some health care providers to be safe, even though there is no scientific basis for this belief [6].
Trichosanthes cucumerina Linn. is one of the numerous herbs that are of high medicinal value [7]. It is an annual, dioecious climber belonging to the family cucurbitaceae found in the wild across much of South and Southeast Asia, and Southern China, Northern Australia and naturalized in Florida, parts of Africa including Nigeria and on various islands in the Indian and Pacific Oceans [8]. The soft-skinned immature fruit can reach up to 150cm (59in) in length and its soft, bland, somewhat mucilaginous flesh is similar to that of the luffa and the calabash [9]. It is popular in the cuisines of South Asia and grown in some home gardens in Africa with the fruit usually consumed as a vegetable due to its good nutritional value [10]. Trichosanthes cucumerina L, is utilized as a substitute to the solanaceous tomato (Lycopersicon esculentum (L.) Mill.) due to its sweet tasting, aromatic, and deep red endocarp pulp when fully ripe, the fruit pulp does not go sour as quickly as paste gotten from l. esculentum [11]. Fruits and vegetables are good sources of natural antioxidants for the human diet, containing many different antioxidan0t components which provide protection against harmful free radicals and have been strongly associated with reduced risk of chronic diseases such as cardiovascular disease, cancer, diabetes, Alzheimer’s disease, cataracts and age-related functional decline in addition to other health benefits [8-11]. Because if the antioxidant property, its role in neurological diseases including Alzheimer’s disease has been proposed [7,12,13]. It reduces the stress in the brain, calms the nervous system and can be used to target specific brain areas to treat neurological diseases [12].
The cerebellum is the region of the brain that plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language, and in regulating fear and pleasure responses but its movement related functions are the most solidly established [14-16]. The cerebellum does not initiate movement, rather it contributes to coordination, precision, and accurate timing. Cerebellar injures have been reported to result from toxins, auto antibody and structural lesion [17]. Since there is little information in the literature regarding the usage of Trichosanthes cucumerina in especially its effect on cerebellar neurons, the current study examines the effect of the extract from Trichosanthes cucumerina on the cerebellum using adult Wistar rats as models over a period of twenty-eight days. We tested the hypothesis of extracts of Trichosanthes cucumerina leaf on the cerebellum for a period of twenty-eight (28) days will provide neuroprotective function without visible pathology. To test this hypothesis, we determine the histopathological effect of Trichosanthes cucumerina leaf extracts on the cerebellum of adult Wistar rats, by assessing the cytoarchitectural alterations on the cerebellum following administration of methanolic extract of Trichosanthes cucumerina leaf on Wistar rats.
Material and Methods
Preparation and storage of extracts from Trichosanthes cucumerina
Figure 1: An intact Trichosanthes cucumerina.

Trichosanthes cucumerina leaves (Figure 1) used for this study is collected from Ndiya farm of Nsit Ubium local government Area of Akwa Ibom State, Nigeria. It is identified and authenticated by the curator of Department of Botany Herbarium, University of Calabar, and a voucher specimen number (TS#10) is deposited at the Department Herbarium. The leaves are harvested manually, washed, dried under shade for four weeks under ambient temperature of 30°-40 °C and is thereafter grounded into fine powder, packed into airtight containers.
The preparation of methanolic extract of Trichosanthes cucumerina is carried out at the Department of Chemistry Research Laboratory, University of Calabar, using methanolic solvents for duration of 72 hours. Forty grams (40g) of the powdered leaf is being soaked in 100ml of methanol and each part of the grounded Trichosanthes cucumerina leaf is boiled in distilled water for 15minutes, filtered and then cooled with muslin cloth after which the filtrate is concentrated using the rotary evaporator. The solvent is then removed by pouring the filtrate into an evaporating dish and placed in a water bath at 80 °C at reduced pressure of about 70%HHmg. The extract is collected and stored in a dry glass container at a temperature of 0-4 °C. Fresh solution of the extract is prepared in distilled water just before use to maintain its potency.
Experimental animals
Cages: Cages made from plastic rubbers with wire mesh covers are used to house the rats. Feed is given in constructed cemented troughs to minimize spoilage and spillage; while water is provided in plastic containers fitted with stainless steel nozzles and given liberally. Cages are placed away from windows to avoid direct sunlight on the animals. The rats are fed with grower’s mash from Vital Feeds obtained from A.B Henshaw Stores at No. 53 Mount Zion Road, Calabar.
Breeding of animals: Thirty-two (32) healthy adult female albino Wistar rats with average of 200g are used for the experiment. The animals are procured from the animal house of Department of pharmacology University of Calabar and is housed in raised mesh bottom cages under standard animal house conditions and fed with standard rat feed and water ad libitum after acclimatizing them for 14 days. All animal experiment is conducted in accordance with the internationally accepted laboratory animal use and care, guidelines and rules of the ethical committee for animal experimentations.
Administration of extract: A total of thirty-two adult Wistar rats of average weight 200g are used for this experiment. Random sampling is used to divide the rats into four Groups (A, B, C & D) of 8 animals each. Group A serve as the experimental control and receive feed and distilled water ad libitum. Experimental Group B, C and D receive 200mg/kgBW, 400mg/kgBW and 600mg/kgBW of methanolic leaf extract of Trichosanthes cucumerina and also given feed and distilled water ad libitum. 1/7th mg,1/3.5th mg and 1/2.3thmg of the effective lethal dose of 1400mg/kg per kgBW of the plant extract is administered orally to the experimental rats with the aid of an orogastric tube after dissolving in distilled water. Treatment of experimental rats with methanolic leaf extract of Trichosanthes cucumerina lasted for a duration of 28 days.
Termination of experiment: Twenty-four hours after the last day of extract administration, the animals are anaesthetized using chloroform vapor in a closed chamber and are sacrificed. The cerebellum is excised and fixed in 10% formalin for histopathological studies and tissue processing.
Tissue processing
Following fixation, dehydration is done by passing the tissue via ascending grades of alcohol: 70% alcohol for two hours each and two changes of 100% alcohol for two hours each. Clearing which is the next step is carried out by transferring the tissue to equal volume of xylene as absolute alcohol. This is done to avoid a shard change from alcohol to xylene which may lead to the distortion of the tissue. The tissue is then infiltrated with wax. Infiltration is done twice at 58 °C for one hour each in each procedure.
Embedding follows suit by putting the tissue in an embedding mould in molten paraffin wax. The tissue is then transferred to the mould by using warm forceps and holding it gently by placing the cut side down. The mould is transferred to the cold plate and gently the tissue is pressed flat. The surface of the mould are trimmed of to allow only 3mm paraffin wax thick to surround the tissue. The paraffin blocks are then mounted on wooden block with an acid led spatula and allowed to solidify for about 30 minutes. Sectioning is done using rotatory microtome in ribbon section of 5mm thickness. The water bath is turned on and the temperature adjusted to about 35-37 °C. The blocks are placed face down on an ice block or heat sink for ten minutes. A fresh blade is placed on the microtome and the block inserted into the microtomes. The desired sections are cut out and floated in a water bath containing warm water and mounted on the slides rubbed with egg albumin as adhesive. The slides are then dried in the oven at 60 °C before staining.
Staining (Haematoxylin and Eosin method)
The staining process is carried out using staining trough and racks. Xylene is poured into the staining trough and the racks containing the slides are immersed in 2 changes of xylene for 8-10minutes each. Immediately after the last xylene change; the staining trough and the slides are transferred to absolute alcohol of 2 changes for 3-5 minutes each. This is 3-5 followed by 95% alcohol for 2 changes and 70% both for3-5 minute to remove xylene completely from the tissue sections.
The sections are then washed in running tap water for 5minutes. After this, the sections are stained with haematoxylin then washed in running tap water for 15minutes. Differentiation of sections is carried out with 1% acid alcohol for 1 second. This is done as quickly as possible to prevent the acid alcohol from damaging the stained slides completely. The tissues are counterstained with eosin, to produce a contrast background to the staining of the components of the tissues. Haematoxylin stain the nuclei of the cells blue and eosin stain cytoplasm of the cell pink. The sections are then passed through ascending grades of alcohol for 2 changes each for 3 minutes. This is done to dehydrate sections. The dehydrated sections were then passed through 2 changes of xylene for 3 minutes each to clear the alcohol and then mounted on slides using Distrene Trieresy Phosphate xylene (DPX) mountant.
Results
As shown in the control groups (Figure 2 & 3) the cerebellar cortex show intact layers composed an outer hypocellular molecular layer, middle Purkinje cell layer, and inner densely populated granular cell layer. The molecular layer consists of scanty deeply stained neuronal cell bodies and abundant neuronal processes. The purkinje cells are prominent with abundant cytoplasm and a distinct nucleus with conspicuous nucleoli. The granular cell layer consists of uniform deeply stained small neuronal cells with granular cytoplasm.
Figure 2: CONTROL X100.
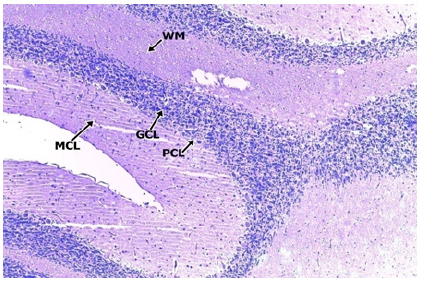
Figure 3: CONTROL GROUP X400.
Photomicrographs of the cerebellum of the Control Group showing normal histological appearance. H&E (x100) (x400) WM: White Matter; MCL: Molecular Cell Layer, PCL: Purkinje Cell Layer; PC: Purkinje Cell; GCL: Granular Cell Layer; GC: Granular.
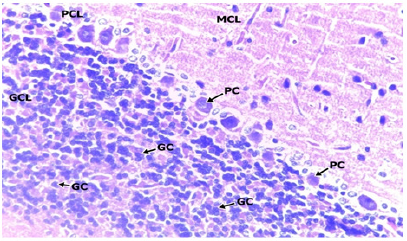
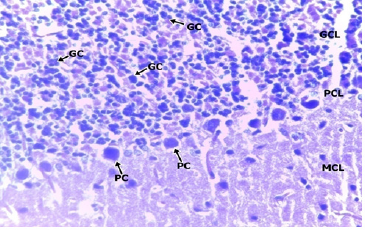
In the low dose group administered with 200mg/kgBW of methanolic extract of TC (Figure 3 & 4), a section of the cerebellar cortex shows intact layers composing of an outer hypocellular molecular layer, middle Purkinje cell layer, and inner densely populated granular cell layer. The purkinje cells have prominent nuclei with nucleoli and the surrounding cytoplasm is moderate. The cells of the granular cells are prominent with small round to oval densely stained nuclei. The molecular cell layer consists of sparsely populated neuronal cell bodies and processes. There is no difference when compared to the Control (Figure 4 & 5).
Figure 4: LOW DOSE x100.

Figure 5: LOW DOSE x400.
Photomicrographs of the cerebellum of the Low dose Group administered with 200mg/kg BW of methanolic extract of TC. H&E (x100) (x400) WM: White Matter; MCL: Molecular Cell Layer, PCL: Purkinje Cell Layer; PC: Purkinje Cell; GCL: Granular Cell Layer; GC: Granular Cell.
Figure 6: MEDIUM DOSE x100.
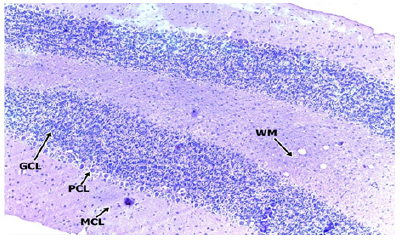
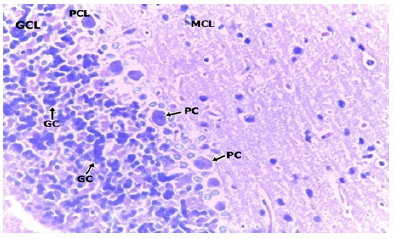
In the medium dose group, 400mg/kgBW, a section of the cerebellar cortex shows intact layers composed of an outer hypocellular molecular layer, middle Purkinje cell layer, and inner densely populated granular cell layer. The purkinje cells are prominent with distinct nuclei and nucleoli. The granular cell layer consists of densely populated small neuronal cells. No cell degeneration noted (Figure 6 & 7). In the high dose group (group D) administered with 600mg/kgBW, a section of the cerebellum shows a prominent cerebellar cortex consisting of an intact molecular, purkinje and granular cell layer. The molecular cell layer consists of sparsely populated neuronal cell bodies and processes. The purkinje cells are prominent with regular cytoplasmic and nuclei outline having a prominent nucleolus. The granular cell layer is dense and comprises of small uniform cells with granular cytoplasm and deeply stained nuclei. No pathology seen (Figure 8 & 9).
Figure 7: MEDIUM DOSE x400.
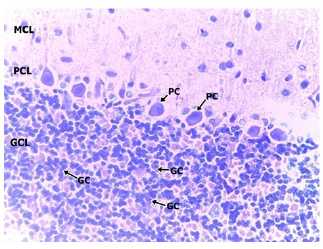
Photomicrographs of the cerebellum of Medium dose Group administered with 400mg/kg BW of methanolic extract of TC. H&E (x100) (x400); WM: White Matter; MCL: Molecular Cell Layer; PCL: Purkinje Cell Layer; PC: Purkinje Cell; GCL: Granular Cell Layer; GC: Granular Cell.
Figure 8: HIGH DOSE x100.
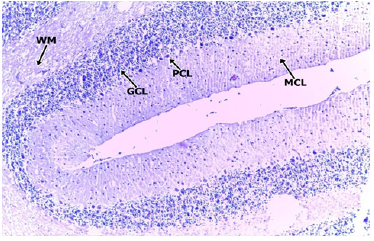
Figure 9: HIGH DOSE x400.
Photomicrographs of the cerebellum of the High dose Group administered with 600mg/kg BW of methanolic extract of TC.
H&E (x100) (x400) WM: White Matter; MCL: Molecular Cell Layer; PCL: Purkinje Cell Layer; PC: Purkinje Cell; GCL: Granular Cell Layer; GC: Granular Cell.
Discussion
In the present study, administration of varying doses of methanolic extract of Trichosanthes cucumerina extract for 28 days shows no histopathological alterations in the cerebellum of all treated group of rats; they retain their normal cytoarchitecture when compared to the normal control. The cortical layers; molecular layer, purkinje cell layer, granular layer and white matter appear distinct and normal in all with no vacuolations or degenerations. The histology of rats in the low does group shows no difference when compared to the control. This indicates that at 200mg/kgBW, TC is safe for administration. In the medium dose group (400mg/kgBW), the histology of the cerebellum shows intact cell layers. There is no form of cell degeneration or vacuolation, indicating that TC may stall or hinder proliferation of cells. In general, the results from the present study show that in all three experimental groups, there is no pathology observed. This may indicate protection functions probably due to the predominant presence of cucurbitacins found in cucurbitaceae and several other families of the plant kingdom [18,19]. Cucurbitacins are tripenoid compounds found in TC, and originally identified as the bitter components of the cucurbit family that demonstrated several pro-survival activities in various model of cellular decay [20-25]. Cucurbitacins, phytosterols found mostly in cucurbits, and are currently being studied for their anticancer functions [21-25]. This occurs because cucurbitacins binds with actin resulting in the stabilization microfilaments [26], arrest cell cycle and apoptosis in rapidly dividing cells, such as tumor [27]. In addition, cucurbitacin is known to suppress cell invasion and metastasis [28] and does not affect apoptosis in human lymphocytes as compared to prostate adenoma and breast cancer cell lines [29].
A recent study demonstrated the neuroprotective properties of cucurbitacins on dopaminergic neurons [30]. The autophagylysosomal degradation pathway, which is essential for turnover of mitochondria and degradation of aggregated proteins was associated with this terpenoid molecule and its capability for neuroprotection [31-33]. Autophagy impairment has been reported in many neurodegenerative disorders [34,35], and the autophagylysosome pathway has been proposed for neurodegeneration therapies [36-40]. It is also important to point out that autophagy activation has been shown to be detrimental in other models of neuronal loss such as stroke [41-43]. Therefore, while autophagy activation can promote survival when the accumulation of damaged cellular components is the primary issue, it has a detrimental effect on neurons. In this context, it is possible that the neuroprotective effects of cucurbitacins in a neuronal model of cell death are associated with a decrease in autophagy. Our results do not exclude the possibility that the neuroprotective effects of cucurbitacins in TC may be related to a stabilizing action exerted on the neuronal cytoskeleton. This is maybe associated with the modulation of the autophagy pathways associated with lysosomal activities for this terpenoid molecule in the neuroprotection of the cerebellum neurons.
Conclusion
Natural molecules in leaves of Trichosanthes cucumerina have some potential neuroprotective roles in the cerebellum. We evaluated the neuroprotective potential of leaves of Trichosanthes cucumerina with a rich content of cucurbitacin. We found that administration of ascending grades of methanolic extract of Trichosanthes cucumerina (200, 400, 600mg/kgBw) to adult female Wistar rat resulted in no histopathological effect in the cerebellum with no alterations in the cortical layers. It is possible that the presence of biologically active phytochemicals in the plant may have a neuro-protective effect especially since the dosage given in the entire treatment group crossed the blood brain barrier and the dosage given did not produce any effect.
References
- Thomford NE, Senthebane DA, Rowe A, Munro D, Seele P, et al. (2018) Natural products for drug discovery in the 21st Century: Innovations for novel drug discovery. International Journal of Molecular Sciences 19(6).
- Pan SY, Litscher G, Gao SH, Zhou SF, Yu ZL, et al. (2014) Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources. Evidence-Based Complementary and Alternative Medicine 2014.
- Hahn-Deinstrop E, Koch A, Muller M (1998) Guidelines for the assessment of the traditional herbal medicine 'Olibanum' by application of HPTLC and DESAGA ProViDoc((R)) video documentation. JPC-Journal of Planar Chromatography-Modern Tlc 11: 404-410.
- Thomford NE, Dzobo K, Chopera D, Wonkam A, Skelton M, et al. (2015) Pharmacogenomics implications of using herbal medicinal plants on african populations in health transition. Pharmaceuticals 8(3): 637-663.
- Mohammed TA, Al Bassir RM (2016) Herbal medicine today: Clinical and research issues. Journal of Thoracic Oncology 11: S80.
- Firenzuoli F, Gori L (2007) Herbal medicine today: Clinical and research issues. Evidence-Based Complementary and Alternative Medicine 4: 37-40.
- Arawwawala M, Thabrew I, Arambewela L, Handunnetti S (2010) Anti-inflammatory activity of Trichosanthes cucumerina in rats. Journal of Ethnopharmacology 131: 538-543.
- Rudroju S, Gudikandula K, Talari S, Nanna RS (2016) Antibacterial activity of different extracts of Trichosanthes cucumerina l an endangered ethnomedicinal herb. International Journal of Pharmaceutical Sciences and Research 7: 1093-1102.
- Bamidele P, Fasogbon MB (2017) Chemical and antioxidant properties of snake tomato (Trichosanthes cucumerina) juice and Pineapple (Ananas comosus) juice blends and their changes during storage. Food Chemistry 220: 184-189.
- Nagendran K, Aravintharaj R, Mohankumar S, Manoranjitham SK, Naidu RA, et al. (2015) First report of cucumber green mottle mosaic virus in snake gourd (Trichosanthes cucumerina) in India. Plant Disease 99: 559.
- Pradeep K, Pani DR, Bhatt KC (2015) Taxonomic notes on the Trichosanthes cucumerina group (Cucurbitaceae) from India. Novon 24: 39-45.
- Adebooye OC (2008) Phyto-constituents and anti-oxidant activity of the pulp of snake tomato (Trichosanthes cucumerina L.). African Journal of Traditional Complementary and Alternative Medicines 5: 173-179.
- Arawwawala M, Thabrew I, Arambewela L (2011) Evaluation of the toxic potential of standardized extracts (hot water extract and cold ethanolic extract) of Trichosanthes cucumerina aerial parts. Boletin Latinoamericano Y Del Caribe De Plantas Medicinales Y Aromaticas 10: 11-22.
- Heleven E, van Dun K, Van Overwalle F (2019) The posterior Cerebellum is involved in constructing Social Action Sequences: An fMRI Study. Scientific Reports 9.
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, et al. (2014) Consensus paper: The cerebellum's role in movement and cognition. Cerebellum 13: 151-177.
- Koziol LF, Budding DE, Chidekel D (2012) From movement to thought: Executive function, embodied cognition, and the cerebellum. Cerebellum 11: 505-525.
- Jang SH, Kwon HG (2019) Improvement of ataxia in a patient with cerebellar infarction by recovery of injured cortico-ponto-cerebellar tract and dentato-rubro-thalamic tract: a diffusion tensor tractography study. Neural Regeneration Research 14: 1470-1472.
- Bourebaba L, Gilbert-Lopez B, Oukil N, Bedjou F (2020) Phytochemical composition of Ecballium elaterium extracts with antioxidant and anti-inflammatory activities: Comparison among leaves, flowers and fruits extracts. Arabian Journal of Chemistry 13: 3286-3300.
- Zhong YL, Xu H, Zhong Y, Zhang XM, Zeng T, et al. (2019) Identification and characterization of the Cucurbitacins, a novel class of small-molecule inhibitors of Tropomyosin receptor kinase. Bmc Complementary and Alternative Medicine 19.
- Attard E, Martinoli MG. Cucurbitacin E (2015) An experimental lead triterpenoid with anticancer, immunomodulatory and novel effects against degenerative diseases. A mini-review. Current Topics in Medicinal Chemistry 15: 1708-1713.
- Bernard SA, Olayinka OA (2010) Search for a novel antioxidant, anti-inflammatory/analgesic or anti-proliferative drug: Cucurbitacins hold the ace. Journal of Medicinal Plants Research 4: 2821-2826.
- Cai Y, Fang XF, He CW, Li P, Xiao F, et al. (2015) Cucurbitacins: A systematic review of the phytochemistry and anticancer activity. American Journal of Chinese Medicine 43: 1331-1350.
- Chen XP, Bao JL, Guo JJ, Ding Q, Lu JJ, et al. (2012) Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anti-Cancer Drugs 23: 777-787.
- Habib L, Jraij A, Khreich N, Fessi H, Charcosset C, et al. (2014) Morphological and physicochemical characterization of liposomes loading cucurbitacin E, an anti-proliferative natural tetracyclic triterpene. Chemistry and Physics of Lipids 177: 64-70.
- Liang J, Chen D (2019) Advances in research on the anticancer mechanism of the natural compound cucurbitacin from Cucurbitaceae plants: a review. Traditional Medicine Research 4: 68-81.
- Sorensen PM, Iacob RE, Fritzsche M, Engen JR, Brieher WM, et al. (2012) The natural product Cucurbitacin E inhibits depolymerization of actin filaments. Acs Chemical Biology 7: 1502-1508.
- Sun JZ, Blaskovich MA, Jove R, Livingston SK, Coppola D, et al. (2005) Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene 24: 3236-3245.
- Zhang T, Li JJ, Dong YM, Zhai D, Lai L, et al. (2012) Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion. Breast Cancer Research and Treatment 135: 445-458.
- Dong YM, Lu BB, Zhang XL, Zhang J, Lai L, et al. (2010) Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 31: 2097-2104.
- Arel-Dubeau AM, Longpre F, Bournival J, Tremblay C, Demers-Lamarche J, et al. (2014) Cucurbitacin E has neuroprotective properties and Autophagic modulating activities on dopaminergic neurons. Oxidative Medicine and Cellular Longevity 2014.
- Rubinszfein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, et al. (2005) Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy 1: 11-22.
- Chu CT (2006) Autophagic stress in neuronal injury and disease. Journal of Neuropathology and Experimental Neurology 65: 423-432.
- Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. Journal of Cell Science 120: 4081-4091.
- Yang F, Yang YP, Mao CJ, Cao BY, Cai ZL, et al. (2009) Role of autophagy and proteasome degradation pathways in apoptosis of PC12 cells overexpressing human alpha-synuclein. Neuroscience Letters 454: 203-208.
- Xilouri M, Brekk OR, Kirik D, Stefanis L (2013) LAMP2A as a therapeutic target in Parkinson disease. Autophagy 9: 2166-2168.
- Pan TH, Kondo S, Le WD, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain 131: 1969-1978.
- Jimenez-Moreno N, Stathakos P, Caldwell MA, Lane JD (2017) Induced pluripotent stem cell neuronal models for the study of autophagy pathways in human neurodegenerative disease. Cells 6.
- Rui YN, Le WD (2015) Selective role of autophagy in neuronal function and neurodegenerative diseases. Neuroscience Bulletin 31: 379-381.
- Son JH, Shim JH, Kim KH, Ha JY, Han JY (2012) Neuronal autophagy and neurodegenerative diseases. Experimental and Molecular Medicine 44: 89-98.
- Yue ZY, Friedman L, Komatsu M, Tanaka K (2009) The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochimica Et Biophysica Acta-Molecular Cell Research 1793: 1496-1507.
- Gao L, Jiang T, Guo J, Liu Y, Cui GY, et al. (2012) Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PloS One 7.
- Qin HD, Tan WG, Zhang Z, Bao L, Shen H, et al. (2015) 15d-Prostaglandin J(2) Protects Cortical Neurons Against Oxygen-Glucose Deprivation/Reoxygenation Injury: Involvement of Inhibiting Autophagy Through Upregulation of Bcl-2. Cellular and Molecular Neurobiology 35: 303-312.
- Xu F, Li J, Ni W, Shen YW, Zhang XP (2013) Peroxisome proliferator-activated receptor-gamma Agonist 15d-Prostaglandin J(2) mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PloS One 8.
© 2020 Utibe Udonwa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






