- Submissions

Full Text
Advances in Complementary & Alternative medicine
Antioxidant and Anti-inflammatory Activities of the Biofield Energy Healing Based Proprietary Test Formulation in Unpredictable Chronic Stress (UCS) induced Sprague Dawley Rats Model
Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1, Gopal Nayak1 and Snehasis Jana2*
1Trivedi Global, Inc., USA
2Trivedi Science Research Laboratory Pvt Ltd, India
*Corresponding author:Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd. Thane (W), India
Submission: January 23, 2020;Published: February 14, 2020

ISSN: 2637-7802 Volume5 Issue5
Abstract
The oxidative stress plays an important role in the pathogenesis of anxiety and depression. Antioxidant assay was performed in brain tissue to evaluate the effect of Consciousness Energy Healing Treatment (the Trivedi Effect®) on a novel test formulation in male Sprague Dawley (SD) rats along with pro-inflammatory cytokines estimation using ELISA assay. The test formulation included the combination of minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, alpha tocopherol, cyanocobalamin, and cholecalciferol), Panax ginseng extract, β-carotene, and cannabidiol isolate. All the constituents of the test formulation were divided into two parts; one portion was defined as the untreated test formulation, while the other portion of the test formulation and the animals received Biofield Energy Healing Treatment by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. Antioxidant assay dada showed that superoxide dismutase (SOD) level was increased by 50.9%, 18.4%, 47.6%, 24%, and 27.4% in the G5 group, which was received Biofield Energy Treated Test formulation, Biofield Energy Treatment per se to the rats (G6), 15-days pre-treatment of the Biofield Energy Treated test formulation (G7), 15-days pre-treatment of Biofield Energy Treated test formulation to the Biofield Energy Treatment per se to rats (G8), and untreated test formulation to the Biofield Energy Treated rats (G9) groups, respectively as compared with the disease control group (G2). The level of lipid peroxidation (LPO) in the brain was significantly decreased by 20%, 21.5%, and 10% in the G5, G6, and G9 groups, respectively as compared with the disease control group (G2). However, IL-6 level in the brain was significantly decreased by 35.9%, 18.2%, 10.5%, 26.9%, and 32.6% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2. The TNF-α level in brain was decreased by 73.3%, 57.2%, 63%, 80.5%, and 72.7% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2. The IL-1ß level in brain was decreased by 40.2%, 37.8%, 26.7%, 43.5%, and 49.9% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2. Overall, the data suggested that there was significant antioxidant and anti-inflammatory effect observed for the Biofield Energy per se along with preventive maintenance groups in the preclinical rat model study. Therefore, the results showed the significant slowdown the stress related disease progression and its complications/symptoms in the preventive Biofield Energy Treatment group per se and/or Biofield Energy Treated Test formulation groups (viz. G6, G7, G8, and G9) comparatively with the disease group (G2). This energy treatment (the Trivedi Effect®) might also be beneficial against various oxidative stress-related disorders (Parkinson's disease, Alzheimer's disease, diabetes, and cardiovascular conditions such as high blood pressure, atherosclerosis, and stroke, etc.) and inflammatory disorders (asthma, autoimmune diseases, coeliac disease, glomerulonephritis, hepatitis, inflammatory bowel disease, etc.).
Keywords: Biofield treatment; Antioxidant assay; Brain cytokines; The trivedi effect®; Unpredictable chronic stress; ELISA
Introduction
Stress could be evoked by any physiological, environmental, or psychological strain that basically poses a threat to the body’s homeostasis. Besides, the nature of stress might change dramatically along with the development of science and technology, as well as the economical status and social competition. Such stressful events might cause severe alterations in the neurotransmitters, neurochemicals, and other hormones by affecting the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis [1]. Moreover, chronic stress is considered as a major risk factor regarding the development of several psychiatric disorders; such as, major depressive disorder (MDD) and their maintenance. The exposure to chronic stress may alter the neuronal morphology as well as function across multiple brain regions, such as, hippocampus, prefrontal cortex, and amygdale [2]. Although several studies have reported the occurrence of neuronal damage and depression due to chronic stress, however, the exact mechanism still remains unknown. It is anticipated that since, major part of neuronal cell membrane is polyunsaturated fatty acids; they become vulnerable to lipid peroxidation-induced damage by acting as the substrate for reactive oxygen species. In normal conditions, the endogenous antioxidant defense systems act by eliminating the produced reactive oxygen species, but the deficient antioxidant defense can cause the oxidative stress-induced damage [3]. The antioxidant defense systems manage the physiologically optimal levels of ROS in the body with the help of antioxidant enzymes (AOEs) such as, cellular and mitochondrial superoxide dismutases, catalase (CAT), glutathione reductase (GLR), and glutathione peroxidase (GPx); as well as some non-enzymatic antioxidants such as glutathione (GSH) [4]. These antioxidant enzymes form the first line of defense against free radicals; hence, the regulation basically depends upon the oxidant status of the cell. Besides, there are some other factors that plays major role in their regulation, such as the enzyme-modulating action of various hormones including prolactin, growth hormone, and melatonin. Such factors may also stimulate various antioxidant enzymes by increasing their activity or by stimulating the gene expression for these enzymes [5]. It was observed that the intense stressors may also over-activate the immune system, which further causes the imbalance of the inflammation and anti-inflammation. Various scientific studies reported the induced pro-inflammation by stress, including IL-6, C-reactive protein (CRP), IL-1β, TNFα, and the transcription factor of NF-κB [6,7]. Besides, there may be central inflammation namely neuro-inflammation also been found in stress condition along with the peripheral inflammation [8,9]. The research studies reported the increased microglia activation, elevated pro-inflammatory cytokines, and accumulation of peripherally derived monocytes and macrophages in the brain after the psychological stress exposure [10]. Such activation of microglia display hypertrophic branch morphology with an enlarged soma and it also produce an exaggerated cytokine to recruit peripheral monocytes. Similarly, the increased brain macrophages and circulating monocytes may elevate the production of pro-inflammatory cytokine (IL-1β, TNFα, IL-6) and their levels in the brain [11]. To evaluate the effect on brain antioxidant and cytokines, novel test formulation was designed, comprised of vital minerals (selenium, zinc, iron, calcium, copper, and magnesium), essential vitamins (cyanocobalamin, ascorbic acid, pyridoxine HCl, alpha tocopherol, and cholecalciferol), and nutraceuticals (β-carotene, Ginseng, cannabidiol isolate (CBD)). In addition, the test formulation was treated with the complementary medicine, Biofield Energy Treatment by a renowned Biofield Energy Healer.
Biofield Energy Healing therapy is considered as the Complementary and Alternative Medicine (CAM) treatment, which has been found with significant therapeutic benefits. Biofield Energy therapy has been reported to be useful against many immune disorders and a clinical case report of cervical cancer patients found with significant benefits as compared with the conventional treatment [12]. National Center for Complementary/Alternative Medicine (NCCAM) has defined and recommended the CAM therapies, which has been exist in various forms of therapies like external qigong, Johrei, Reiki, therapeutic touch, yoga, Qi Gong, polarity therapy, Tai Chi, pranic healing, deep breathing, chiropractic/osteopathic manipulation, guided imagery, meditation, massage, homeopathy, hypnotherapy, progressive relaxation, acupressure, acupuncture, special diets, relaxation techniques, Rolfing structural integration, healing touch, movement therapy, pilates, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems both in vitro and in vivo. In addition, Biofield Energy has been found to have its subtle energy, which has the capacity to work in an effective manner [13] with its various clinical benefits [14]. Biofield Energy Treatment (the Trivedi Effect®- Consciousness Energy Healing Treatment) has been studied and were reported with numerous significant outcomes in the field of pharmaceuticals [15-17], nutraceuticals [18,19], metals and ceramics [20-22], microbiology [23-25], microbial genetics [26,27], cancer research [28,29], livestock, and agriculture science [30-32]. Thus, the present work was designed to study the effect of the Biofield Energy Treatment (the Trivedi Effect®) on the given novel test formulation and Biofield Energy Treatment per se to the animals brain tissue for the estimation of antioxidant level and cytokines assay in presence of Unpredictable Chronic Stress (UCS) induced Sprague Dawley rats model using standard ELISA assay.
Material and Methods
Chemicals and reagents
Pyridoxine hydrochloride (vitamin B6), calcitriol, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, Provit A) were purchased from TCI, Japan. Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (Alpha-Tocopherol), cholecalciferol (vitamin D3), iron (II) sulfate, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma-Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Cannabidiol isolate and Panax ginseng extract were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. Imipramine Hydrochloride was purchased from Sigma, USA. For the estimation of antioxidants and cytokines, specific ELISA kits were used such as for detection of brain antioxidant panel and cytokine assay were procured from Cayman chemical and CUSABIO, USA, respectively.
Maintenance of animal
Randomly breed male Sprague Dawley (SD) rats with body weight ranges from 200 to 300gm were used in this study. The animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India. Animals were randomly divided into nine groups based on their body weights consist of 6 animals of each group. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol throughout the experiment.
Consciousness energy healing strategies
The novel test formulation was subjected to Biofield Energy Healing Treatment, thus each ingredient was distributed into two parts. One part of the test compound did not receive any sort of Biofield Energy Healing Treatment and were defined as the untreated or control sample. The second part of the test formulation was treated with The Trivedi Effect® -Energy of Consciousness Healing Treatment (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for ~3 minutes in the research laboratory of Dabur Research Foundation, New Delhi, India. The novel test formulation was consisted of zinc chloride, iron (ii) sulfate, copper chloride, vitamin B6, vitamin B12, vitamin D3, sodium selenate, calcium chloride, ascorbic acid, vitamin E, beta carotene, Panax ginseng extract, cannabidiol and magnesium (II) gluconate. Besides, three group of animals also received Biofield Energy Healing Treatment (known as the Trivedi Effect®) by Mr. Mahendra Kumar Trivedi under similar laboratory conditions for ~3 minutes. in the research laboratory of Dabur Research Foundation, New Delhi, India. The energy transmission was done without touching the samples or animals. After that, the Biofield Energy Treated samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer under the same laboratory conditions for comparison purposes. The “sham” healer did not have any knowledge about the Biofield Energy Treatment. The Biofield Energy Treated animals were also taken back to experimental room for further proceedings.
Experimental procedure
For experimental procedure, animals were randomized and grouped based on the body weight seven days after acclimatization. G7 and G8 groups dosing were initiated on day -15 and continued till end of the experiment. However, G1 to G5 and G9 groups were dosed from day 1 till the end of experiment. G6 group was not dosed with the test formulation. Body weight and clinical signs were taken daily throughout the experimental period. All the animals except G1 group received stress induced procedures such as stress procedures like sound stress, tilted cages and crowd stress, cold and warm water swim stress, food and water deprivation, stress due to change in the light and dark cycle were undergo seven different types of unpredictable stress procedures after scheduled dosing daily at specified interval to the end of the experiment for 8 weeks after the initiation of stress, which vary every week interval i.e. shuffling of stress type. During 8th week of the experimental period, all the animals were individually subjected for blood collection for the experimental purpose.
Preparation of sample for ELISA assay
With the continued stress treatment of 8th week of the experimental period, all the animals were individually subjected for blood collection using retro-orbital route and the blood was collected in the plain vial in all the animals of different experimental groups. The serum from all the groups was stored at -20 °C for further estimation. Alternatively, aliquot all the samples and store samples at -20 °C or -80 °C. After CSF collection animals were humanely sacrificed to collect brain portion that was homogenized and subjected for various biomarker analysis using suitable ELISA method. Avoid repeated freeze-thaw cycles, which may alter the level of antioxidant and cytokine level in brain during final calculations.
Estimation of antioxidants and cytokines level in brain
The brain homogenate from all the groups was subjected for the estimation of level of antioxidants (SOD, CAT, LPO), cytokines (IL-6, TNF-α, IL-1β). The entire assay and the cytokines panel was estimation using ELISA method as per manufacturer’s recommended standard procedure. This was a quantitative method and the principle was based on the binding of antigen and antibody in sandwich manner assay.
Statistical analysis
The data were represented as mean ± standard error of mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison One-way analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p ≤0.05 was considered as statistically significant.
Result and Discussion
Estimation of superoxide dismutase (SOD) in brain
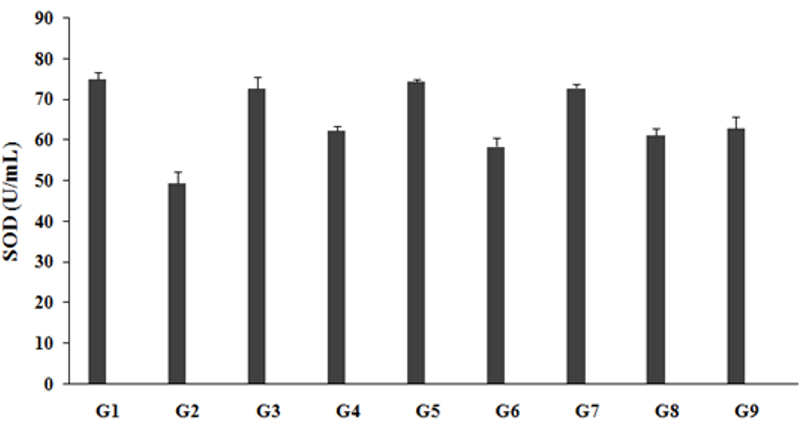
The level of SOD in the unpredictable chronic stress group (G2) was 49.30±2.9U/mL, which was significantly decreased by 34.3% as compared to the normal control (G1, 75.00±1.7U/mL). Imipramine treatment positive control group (G3) showed significantly increased brain SOD level (72.64 ±2.9U/mL) by 47.3% as compared to the G2. However, the untreated test formulation to the untreated rats (G4) showed a significantly increased brain SOD level (62.36±0.9U/mL) by 26.5% as compared to the G2. The Biofield Energy Treated test formulation to the untreated rats (G5) showed a significantly increased brain SOD level (74.42±0.5 U/mL) by 50.9% and 19.3% as compared to the G2 and G4 group, respectively. Biofield Energy Treatment per se to the rats (G6) significantly increased brain SOD level (58.38±2.2U/mL) by 18.4% as compared to the G2. 15-days pre-treatment of the Biofield Energy Treated test formulation (G7) significantly increased brain SOD level (72.77±1.1U/mL) by 47.6% and 16.7% when compared to G2 and G4 group, respectively. 15-days pre-treatment of the Biofield Energy Treated test formulation to the Biofield Energy Treatment per se (G8) significantly increased brain SOD level (61.15±1.7U/mL) by 24.0% as compared to G2. The untreated test formulation to the Energy Treatment per se rats (G9) significantly increased brain SOD level (62.80±2.8U/mL) by 27.4% as compared to the G2. The data suggested significant improved level of SOD, as it is one of the important and strongest antioxidant defense mechanism for all the living cells when they are exposed to oxygen. Reactive oxygen species (ROS) was significantly reduced in presence of SOD enzyme in case of any kind of oxidative stress [33]. Therefore, it is assumed that the Trivedi Effect®-Biofield Energy Treatment based test formulation and Biofield Energy Treatment per se must be a powerful antioxidant in many new therapies for the treatment of inflammatory diseases caused by various forms of stress induction (Figure 1).
Figure 1:The effect of the test formulation on the level of brain antioxidant-SOD in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day-15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
Estimation of antioxidant catalase (CAT) in brain
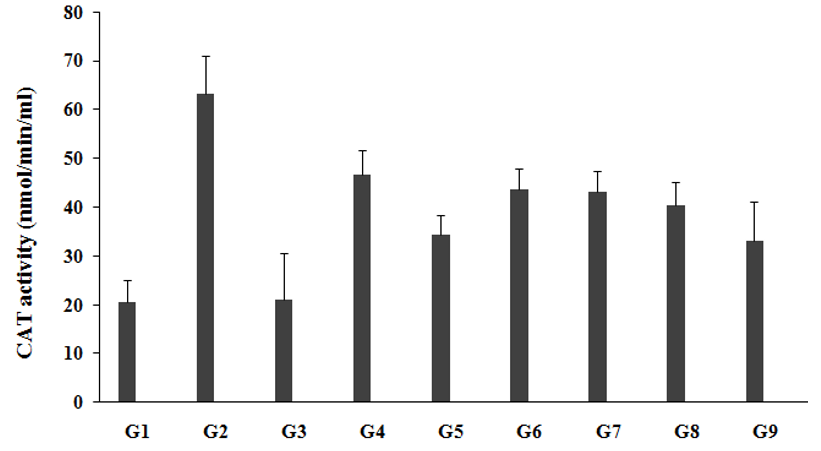
CAT, a scientifically-proven powerful antioxidant enzyme that has very high longevity, which prevent free radical damage to the body. CAT is a powerful antioxidant support, anti-aging, and anti-degenerative effects, enhanced overall lifespan, fat reduction, and prevention of DNA damage due to various stress factors [34]. The effect of the test formulation was compared with respect to the untreated test formulation and Biofield Energy Treatment per se for antioxidant panel in brain. Catalase (CAT) assay in brain was estimated in presence of the effect of test formulation, which was measured in all the experimental groups and was graphically presented in the Figure 2. CAT level in the unpredictable chronic stress group (G2) was 63.23±7.8nmol/min/mL, which was significantly increased by 207.6% as compared to the normal control (G1, 20.55±4.6nmol/min/mL). Imipramine treatment (G3) significantly decreased brain CAT level (21.13±9.6nmol/min/mL) by 66.6% as compared to the G2. The untreated test formulation to the untreated rats (G4) showed decreased brain CAT level (46.68±5.0nmol/min/mL) by 26.2% as compared to the G2. G5 significantly decreased brain CAT level (34.40±4.1nmol/min/mL) by 45.6% and 26.3% as compared to the G2 and G4 groups, respectively. G6 group animals showed significantly decreased brain CAT level (43.63±4.4nmol/min/mL) by 31% and 6.5% as compared to the G2 and G4 groups, respectively. G7 group animals showed significantly decreased brain CAT level (43.05 ±4.5nmol/min/mL) by 31.9% and 7.8% as compared to the G2 and G4 groups, respectively. G8 group animals showed significantly decreased brain CAT level (40.41±4.7nmol/min/mL) by 36.1% and 13.4% as compared to the G2 and G4 groups, respectively. G9 group animals showed significantly decreased brain CAT level (33.09±7.9nmol/min/mL) by 47.7% and 29.1% as compared to the G2 and G4 groups, respectively. Therefore, it is assumed that the Trivedi Effect®-Biofield Energy Treatment based test formulation is a powerful antioxidant agent and worked as free radical scavenging agent against many diseases along with stress induction factors.
Figure 2:The effect of the test formulation on the level of brain antioxidant-CAT in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day-15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
Estimation of antioxidant lipid peroxidation (LPO) in brain
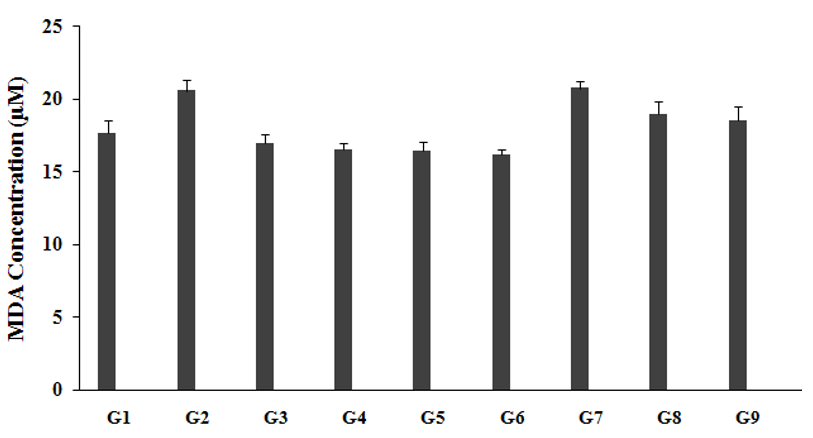
The level of LPO in brain, measured in all the experimental groups and is graphically presented in the Figure 3. LPO level in unpredictable chronic stress group (G2) was 20.54±0.7µM, which was significantly increased by 16.7% as compared to the normal control (G1, 17.60±0.9µM). Imipramine treatment group animals (G3) showed a significantly decreased brain LPO level (16.92±0.7µM) by 17.6% as compared to the G2. The untreated test formulation to the untreated rats (G4) significant decreased brain LPO level (16.47±0.5µM) by 19.8% as compared to the G2. G5 significantly decreased brain LPO level (16.43±0.6µM) by 20% and 0.3% as compared to the G2 and G4 groups, respectively. G6 group animals showed a significantly decreased brain LPO level (16.13±0.4µM) by 21.5% and 2.1% as compared to the G2 and G4 groups, respectively. G7 group animals showed a significantly decreased brain LPO level (20.71±0.5µM) by 0.8% and 25.8% as compared to the G2 and G4 groups, respectively. G8 group animals showed a significantly decreased brain LPO level (18.96±0.8µM) by 7.7% and 15.1% as compared to the G2 and G4 groups, respectively. G9 group animals showed a significantly decreased brain LPO level (18.49±0.6µM) by 10% and 12.3% as compared to the G2 and G4 groups, respectively. Lipid per-oxidation affects lipoproteins, cellular membranes, and other related molecules containing the lipids in accordance with the oxidative stress. The process of LPO was initiated due to many reasons such as acute and chronic stress, cellular damage, altered metabolism, and many more. It leads to various diseases such as inflammatory bowel disease (IBD), atherosclerosis, asthma, kidney damage, retinopathy of prematurity (ROP), Parkinson's disease, borderline personality disorders (BPD), preeclampsia and many more [35-37]. Thus, it is assumed that The Trivedi Effect®-Biofield Energy Treated test formulation and Biofield Energy per se is a powerful antioxidant agent and prevent the oxidation that leads to various stress related disorders.
Figure 3:The effect of the test formulation on the level of brain LPO (reported as MDA concentration) in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day-15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
Estimation of brain cytokine IL-6
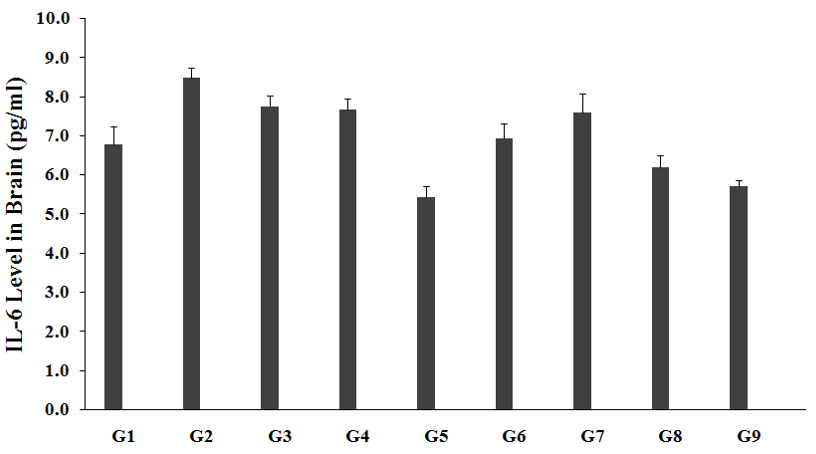
The level of IL-6 in brain was measured in all the experimental groups and are graphically presented in the Figure 4. Brain IL-6 level in unpredictable chronic stress model (G2) was 8.49±0.2pg/mL, which was significantly increased by 25.1% as compared to the normal control (G1, 6.79±0.4pg/mL). Imipramine treatment group (G3) showed a decreased brain IL-6 level (7.76±0.3pg/mL) by 8.6% as compared to the G2. The untreated test formulation to the untreated rats (G4) showed a decreased brain IL-6 level (7.68±0.3pg/mL) by 9.6% as compared to the G2. G5 group animals showed decreased brain IL-6 level (5.44±0.3pg/mL) by 35.9% and 29.1% as compared to the G2 and G4 groups, respectively. G6 group animals showed decreased brain IL-6 level (6.94±0.4pg/mL) by 18.2% and 9.5% as compared to the G2 and G4 groups, respectively. G7 group animals showed decreased brain IL-6 level (7.60±0.5pg/mL) by 10.5% as compared to the G2. G8 group animals showed decreased brain IL-6 level (6.21±0.3pg/mL) by 26.9% and 19.2% as compared to the G2 and G4 groups, respectively. G9 group animals showed decreased brain IL-6 level (5.72±0.1pg/mL) by 32.6% and 25.5% as compared to the G2 and G4 groups, respectively. Cytokine, IL-6 is defined as a soluble mediator with a significant pleiotropic effect on inflammation, immune response, and hematopoiesis [38]. This inflammatory cytokines after treatment was decreased, which suggested relaxation against most of the stress, which regulated the free radicals and many inflammatory diseases. After treatment of the test formulation and Biofield Energy Treatment per se showed similar decreased IL-6 pattern in all the experimental test groups as compared with the G2 and G4 groups. Overall, our results revealed significant decreased values of IL-6 level in brain in all the experimental test groups when compared to G4.
Figure 4:The effect of the test formulation on the level of brain IL-6 in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day-15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
Estimation of brain cytokine TNF-α
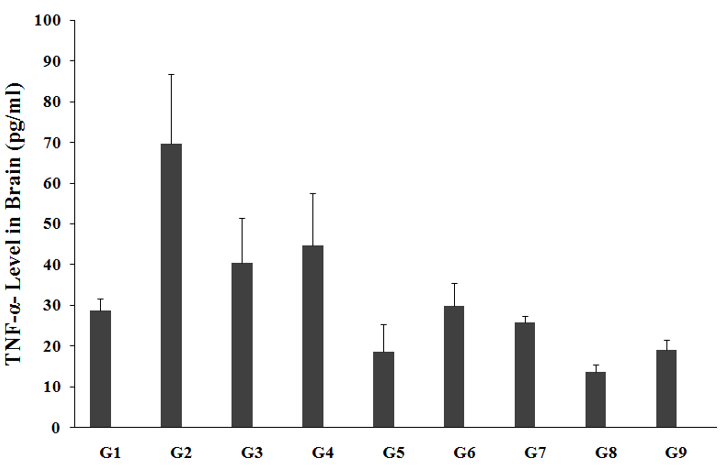
The effect of the test formulation and Biofield Energy Treatment per se was estimated for cytokine, TNF-α level; the results are graphically presented in the Figure 5. Brain TNF-α level in the unpredictable chronic stress model (G2) was 69.74±17.0pg/mL, which was significantly increased by 142.3% as compared with the normal control (G1, 28.78±2.9pg/mL). Imipramine treatment (G3) showed a decreased brain TNF-α level (40.51±10.9pg/mL) by 41.9% as compared to the G2. The untreated test formulation to the untreated rats (G4) showed a decreased brain TNF-α level (44.69±12.9pg/mL) by 35.9% as compared to the G2. G5 group animals showed decreased brain TNF-α level (18.61±6.7pg/mL) by 73.3% and 58.4% as compared to the G2 and G4 groups, respectively. G6 group animals showed a decreased brain TNF-α level (29.86±5.36pg/mL) by 57.2% and 33.2% as compared to the G2 and G4 groups, respectively. G7 group animals showed decreased brain TNF-α level (25.78±1.7pg/mL) by 63% and 42.3% as compared to the G2 and G4 groups, respectively. G8 group animals showed decreased brain TNF-α level (13.61±1.8pg/mL) by 80.5% and 69.5% as compared to the G2 and G4 groups, respectively. G9 group animals showed decreased brain TNF-α level (19.07±2.5pg/mL) by 72.7% and 57.3% as compared to the G2 and G4 groups, respectively. Tumor necrosis alpha (TNF-α) is a classified as a pro-inflammatory cytokine, which has major role in brain inflammation and many other stress related disorders. TNF-α, major pro-inflammatory mediator plays a major role in brain inflammation [39]. It plays a central role in inflammation and immune modulation in various immune-mediated disorders. After treatment with the test formulation and Biofield Energy Treatment per se, the results showed similar decreased TNF-α pattern in all the experimental test groups as compared with the G2 and G4 groups. Overall, our experimental results revealed significant decreased values of TNF-α level in brain in all the experimental test groups when compared to G4.
Figure 5:The effect of the test formulation on the level of brain TNF-α level in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day-15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
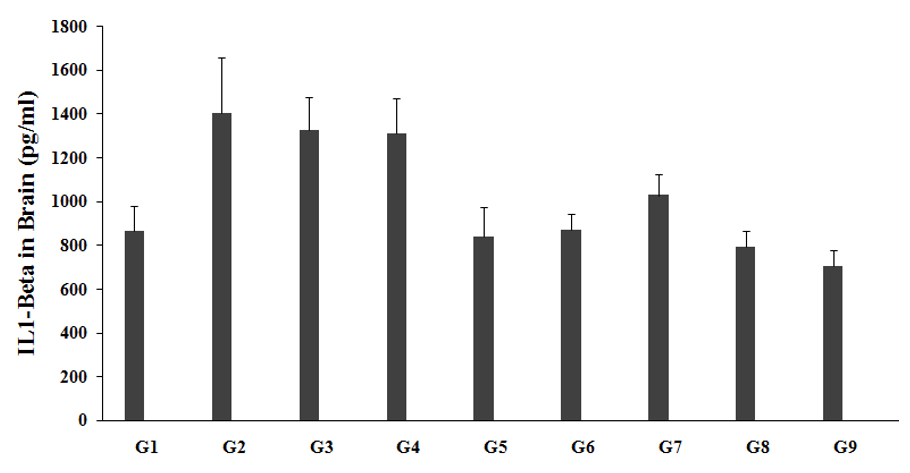
Estimation of brain cytokine IL-1ß
The effect of the test formulation and Biofield Energy Treatment per se was estimated for cytokine, IL-1ß level; the results are graphically presented in the Figure 6. Brain IL-1ß level in the unpredictable chronic stress model (G2) was 1401.99±254.9pg/mL, which was significantly increased by 62.2% as compared with the normal control (G1, 864.59±113.6pg/mL). Imipramine treatment (G3) showed a decreased brain IL-1ß level (1326.53±147.7pg/mL) by 5.4% as compared to the G2. The untreated test formulation to the untreated rats (G4) showed a decreased brain IL-1ß level (1308.24±161.4pg/mL) by 6.7% as compared to the G2. G5 group animals showed decreased brain IL-1ß level (838.86±132.9pg/mL) by 40.2% and 35.9% as compared to the G2 and G4 groups, respectively. G6 group animals showed a decreased brain IL-1ß level (871.39±69.8pg/mL) by 37.8% and 33.4% as compared to the G2 and G4 groups, respectively. G7 group animals showed decreased brain IL-1ß level (1027.10±98.0pg/mL) by 26.7% and 21.5% as compared to the G2 and G4 groups, respectively. G8 group animals showed decreased brain IL-1ß level (792.11±71.2pg/mL) by 43.5% and 39.5% as compared to the G2 and G4 groups, respectively. G9 group animals showed decreased brain IL-1ß level (701.99±74.8pg/mL) by 49.9% and 46.3% as compared to the G2 and G4 groups, respectively. After treatment of the test formulation and Biofield Energy Treatment per se showed similar decreased IL-1ß pattern in all the experimental test groups as compared with the G2 and G4 groups. Overall, our experimental results revealed significant decreased values of IL-1ß level in brain in all the experimental test groups when compared to the G4.
Figure 6:The effect of the test formulation on the level of brain IL-1ß level in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30 mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day-15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
In this research plan, four groups were considered as preventive maintenance groups. These groups were G6 (Biofield Energy Treatment per se to animals at -15 days), G7 (Biofield Energy Treated test formulation from day -15), G8 (Biofield Energy Treatment per se to animals along with Biofield Treated test formulation from day -15), and G9 (Biofield treatment per se at -15 days to animals with untreated test formulation). The results showed the significant slowdown of the disease progression, stress disease related all other symptoms/complications and also reduced the chances of disease susceptibility in these groups. Specifically, group G6 (preventive Biofield Energy Treatment group per se at -15 days) showed the best results as a prophylactic/preventive treatment group compared to the other groups. Based on the overall data, it suggests that the Biofield Energy Healing Therapy was found to be most effective and benefited in order to prevent and protect from the occurrence of any type of diseases in rat model. It indicated that this therapy could act as a preventive maintenance therapy to prevent the occurrence of the disease, slow down the disease progression and disease related complications of the existing aliments that will ultimately improve the overall health and quality of life in human.
Conclusion
Antioxidant assay was estimated using standard ELISA assay along with cytokines estimation in presence of the test formulation. Superoxide dismutase (SOD) level in brain was significantly increased in the Biofield Energy Treated Test formulation to the untreated rats (G5) Biofield Energy Treatment per se to the rats (G6), 15-days pre-treatment of the Biofield Energy Treated test formulation (G7), 15-days pre-treatment of Biofield Energy Treated test formulation to the Biofield Energy Treatment per se to rats (G8), and untreated test formulation to the Biofield Energy Treated rats (G9) groups by 50.9%, 18.4%, 47.6%, 24%, and 27.4%, respectively as compared with the disease control group (G2). Lipid peroxidation (LPO) level in the brain was significantly decreased in the G5, G6, and G9 group by 20%, 21.5%, and 10% respectively, as compared with the G2. However, the level of cytokines such as level of IL-6 in brain was significantly decreased in the G5, G6, G7, G8, and G9 groups by 35.9%, 18.2%, 10.5%, 26.9%, and 32.6% respectively, as compared with the G2. Similarly, the TNF-α level in brain was decreased in the G5, G6, G7, G8, and G9 group by 73.3%, 57.2%, 63%, 80.5%, and 72.7% respectively, as compared with the G2. The IL-1ß level in brain was decreased in the G5, G6, G7, G8, and G9 group by 40.2%, 37.8%, 26.7%, 43.5%, and 49.9% respectively, as compared with the G2. Thus, Biofield Energy Healing Treatment (the Trivedi Effect®) per se showed significance antioxidant activity along with anti-inflammatory activity in the preventive maintenance group, G6 as compared to the other preventive maintenance groups (G7, G8, and G9) in rat model study. The results could be beneficial against various oxidative stress-related disorders (Parkinson’s disease, Alzheimer’s disease, diabetes, and cardiovascular conditions such as high blood pressure, atherosclerosis, and stroke, etc.) and inflammatory disorders (asthma, autoimmune diseases, coeliac disease, glomerulonephritis, hepatitis, inflammatory bowel disease, etc.). It also helped to slow down the disease progression and disease related complications of the overall animal’s health. These data suggested that Biofield Energy Treatment per se and/ or Biofield Energy Treated Test formulation in combination would be the best treatment strategies in order to prevent and protect from the occurrence of any type of diseases. Therefore, the Biofield Energy Treatment might act as a preventive maintenance therapy in order to maintain good health, or full restoration of health or improve the overall health and quality of life in human. This therapy might also reduce the severity of any type of acute/chronic disease (auto-immune related and inflammatory disorders) progression rate and can be used in both before and after the manifestation of any disease symptoms in healthy, unhealthy, and ill peoples such as many thyroid disorders. Altogether, the data suggested the Biofield Energy Treated test formulation and Biofield Energy Treatment per se in showed significant action on thyroid gland with respect to biomarkers, as a Complementary and Alternative Medicine (CAM). This test formulation also can be used against Lupus, Fibromyalgia, Addison Disease, Multiple Sclerosis, Myasthenia Gravis, Aplastic Anemia, Psoriasis, Rheumatoid Arthritis, Crohn’s Disease, Vitiligo, Chronic Fatigue Syndrome and Alopecia Areata, as well as various inflammatory disorders such as Ulcerative Colitis, Dermatitis, Hepatitis, Diverticulitis, Mental Disorders, Stroke, and in the improvement of overall health and quality of life.
Acknowledgement
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
References
- Landsbergis PA (2003) The changing organization of work and the safety and health of working people: A commentary. J Occup Environ Med 45(1): 61-72.
- Maluach AM, Misquitta KA, Prevot TD, Fee C, Sibille E, et al. (2017) Increased neuronal DNA/RNA oxidation in the frontal cortex of mice subjected to unpredictable chronic mild stress. Chronic Stress (Thousand Oaks) 1.
- Che Y, Zhou Z, Shu Y, Zhai C, Zhu Y, et al. (2015) Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci Lett 584: 208-13.
- Djordjevic J, Djordjevic A, Adzic M, Niciforovic A, Radojcic MB (2010) Chronic stress differentially affects antioxidant enzymes and modifies the acute stress response in liver of Wistar rats. Physiol Res 59(5): 729-736.
- Duda W, Curzytek K, Kubera M, Iciek M, Kowalczyk PD, et al. (2016) The effect of chronic mild stress and imipramine on the markers of oxidative stress and antioxidant system in rat liver. Neurotox Res 30(2): 173-184.
- Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65(9): 732-741.
- Liu YZ, Wang YX, Jiang CL (2017) Inflammation: The common pathway of stress-related diseases. Front Hum Neurosci 11: 316.
- Garcia-Bueno B, Caso JR, Leza JC (2008) Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 32(6): 1136-1151.
- Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C, et al. (2008) Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res 41(12): 1037-1046.
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, et al. (2005) Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135(4): 1295-1307.
- Wohleb ES, Delpech JC (2017) Dynamic crosstalk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog Neuropsychopharmacol Biol Psychiatry 79(Pt A): 40-48.
- Turner JG, Clark AJ, Gauthier DK, Williams M (1998) The effect of therapeutic touch on pain and anxiety in burn patients. J Adv Nurs 28(1): 10-20.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S, et al. (2015) Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield. Pharm Anal Acta 6(7).
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Spectroscopic characterization of biofield treated metronidazole and tinidazole. Med Chem 5(7): 340-344.
- Trivedi MK, Branton A, Trivedi D, Shettigar H, Bairwa K, et al. (2015) Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. Nat Prod Chem Res 3(5): 186.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. International Journal of Bioorganic Chemistry 2(3): 135-145.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) Chromatographic and spectroscopic characterization of the consciousness energy healing treated Withania somnifera (ashwagandha) root extract. European Journal of Biophysics 5(2): 38-47.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Characterization of physical and structural properties of aluminum carbide powder: Impact of biofield treatment. J Aeronaut Aerospace Eng 4(1): 142.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Impact of biofield treatment on atomic and structural characteristics of barium titanate powder. Ind Eng Manage 4(3): 166.
- Trivedi MK, Patil S, Nayak G, Jana S, Latiyal O (2015) Influence of biofield treatment on physical, structural and spectral properties of boron nitride. J Material Sci Eng 4(4): 181.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. J Microb Biochem Technol 7(4): 202-205.
- Trivedi MK, Branton A, Trivedi D, Gangwar M, Jana S (2015) Antimicrobial susceptibility, biochemical characterization and molecular typing of biofield treated Klebsiella pneumoniae. J Health Med Inform 6(5): 206.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Antibiogram, biochemical reactions, and genotypic pattern of biofield treated Pseudomonas aeruginosa. J Trop Dis 4(1): 181.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of antibiogram, genotype and phylogenetic analysis of biofield treated Nocardia otitidis. Biol Syst Open Access 4(2): 143.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3(1): 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4(3): 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7(8): 253-257.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Effect of biofield energy treatment on chlorophyll content, pathological study, and molecular analysis of cashew plant (Anacardium occidentaleL.). J Plant Sci 3(6): 372-382.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2016) Molecular analysis of biofield treated eggplant and watermelon crops. Adv Crop Sci Tech 4(1): 208.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Effect of biofield treated energized water on the growth and health status in chicken (Gallus gallus domesticus). Poult Fish Wildl Sci 3(2): 140.
- Banniste J, Bannister W, Rotilio G (1987) Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem 22(2):111-180.
- Djordjevic VB (2004) Free radicals in cell biology. Int Rev Cytol 237: 57-89.
- Vasilaki AT, McMillan DC (2011) Lipid Peroxidation. In: Schwab M (Ed.), Encyclopedia of Cancer. Springer, Berlin, Germany.
- Moller P, Loft S (2010) Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect 118(8): 1126-1136.
- Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R (2008) Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol 153(1): 6-20.
- Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10): a016295.
- Yang T, Li Y, Lyu Z, Huang K, Corrigan CJ, et al. (2017) Characteristics of pro-inflammatory cytokines and chemokines in airways of asthmatics: relationships with disease severity and infiltration of inflammatory cells. Chin Med J (Engl) 130(17): 2033-2040.
© 2020 Mahendra Kumar Trivedi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






