- Submissions

Full Text
Advances in Complementary & Alternative medicine
Micromorphology of Pollen Grains, Trichomes of Sweet Basil, Egypt
Azzazy MF*
Surveys of Natural Resources Department, Egypt
*Corresponding author:Azzazy MF, Surveys of Natural Resources Department, Environmental Studies and Research Institute, Egypt
Submission: April 18, 2019;Published: August 21, 2019

ISSN: 2637-7802 Volume5 Issue1
Abstract
Ocimum basilicum L (sweet basil) belonging to the Lamiaceae family known as the ‘King of Herbs has been used as traditional medicine for various ailments. This study carried out using light and scanning electron microscopy. Anatomy of the Ocimum basilicum L. leaves is characterized by a uniseriate epidermis, sub-epidermal secretory tissue and vascular tissue, while cells contain calcium oxalate crystals. The studied pollen grains were zonocolpate, hexacolpate, and prolate to subprolate. Pollen sculpturing types were recognized perforate, mega reticulate in polar view, with average size 65um. Aperture characterized with stephano-colpate, (hexa-zonocolpate) and margo present. The trichomes were examined, two types of trichomes were recorded, and distinguished non-glandular and glandular trichomes. The non-glandular trichomes appeared valuable data in taxonomy of Lamiaceae while glandular trichomes found short-stalked capitates, small peltate. The peltate trichomes are the most abundant type. The present study demonstrates additional tools for the quality control testing of the medicinal plant sweet basil Ocimum basilicum L. Data obtained revealed characteristic elements of pollen and trichomes of sweet basil (Ocimum basilicum L.) which can be used as a taxonomic character to assure the test of identification of this plant, also to revealed adulteration in herbal mixtures and taxonomic evidence.
Keywords:Pollen grains ultrastructure; Micromorphology; Sweet basil; Trichomes; Anatomy; Lamiaceae
Introduction
Sweet basil (Ocimum basilicum L.) is one of the most popular and healthy culinary herbs in the world. This herb belonging to the Lamiaceae family also referred to as the ‘King of Herbs’ has been used tremendously as traditional medicine for various ailments [1]. Its common name is sweet basil or garden basil, botanical or Latin name is Ocimum basilicum L. its arabic name is Rihaan or Habaq. This plant was used in mummification process in Ancient Egypt as it was mixed with essences of Myrrh and incense. Also, it was also used as an offering to their Gods due to its aromatic fragrance. The biological properties of Ocimum basilicum L. oils which are related to their different interesting applications as antimicrobial, antioxidant, insecticidal and repellent, larvicidal, nematicidal and therapeutic (anti-inflammatory, antipyretic, anti-ulcer, analgesic, anthelmintic, anti-carcinogenic, skin permeation enhancer, immune-modulatory, cardio-protective, anti-lipidemic) agents [2]. Such this herb with therapeutic potential is popularly known as ‘Sweet basil’ which used in both ‘Ayurvedic’ and ‘Unani’ system of medicine while, studies revealed that Ocimum basilicum possesses analgesic, anti-inflammatory, antimicrobial, and cardiac stimulant properties [3]. On the other hand, it is a culinary herb that belongs to the ‘Lamiaceae family’ Sweet basil (Ocimum basilicum L.) belonging to family Lamiaceae, according to [4]. Labiatae (Lamiaceae) is represented in Egypt by 23 genera and 55 species. Ocimum basilicum is known for its antioxidant, antimicrobial, anti-fibrotic and anticancer properties [5]. The aromatic leaves of Ocimum basilicum contain a rich reservoir of phenolic compounds, flavonoids and volatile oils [6]. It is used as a treatment modality for various ailments such as poor digestion, nausea, migraine, depression, insomnia, kidney malfunction and skin infections [7-10] stated that investigations of pollen morphology of the Lamiaceae have been essential as an aid in classification within this family. Palynology has been used considerably in the taxonomy of angiosperms and can be applied in tracing the history of plant groups and species [11]. There is no reported work on the pollen morphology of the genus Ocimum in Egypt. [9] Found in their studies that most of Ocimum species pollen is with bi-reticulate-perforate tectum sculpture and suggested a re-evaluation in their classification. As well as [12] in their study of Ocimum L. species concentrated on pollen colpi reporting different types of pollen a colpate to octacolpate which reveal that there are significant differences among the species. Many species belonging to the family Lamiaceae being highlyaromatic due to presence of external glandular structures, that produce essential oil. The anatomy of leaf showed that, upper and lower epidermis simple, covering with uniseriate trichomes as well as sessile short stalked glandular trichomes [13]. Trichomes are defined as unicellular or multicellular appendages, which originate from epidermal cells [14] and can develop on all parts of the plant: vegetative and reproductive. Type and distribution of trichomes was one of the features differentiating the various subfamilies [15] and [16] found that trichome micromorphology was useful for systematics and reconstructing the phylogeny of Lamiaceae. In some genera of Lamiaceae, the trichome morphology is helpful in infrageneric classification [17]. Inspite of the various medicinal uses attributed to this plant; there are not many pharmacognostical reports on the leaves of this plant in particular. The aim of the present work is to presents the observations on the characteristic elements of pollen and trichomes of sweet basil (Ocimum basilicum L.) which can be used as a taxonomic character to assure the test of identification of this plant specially when used in medical herbal mixtures as powdered form, also to revealed adulteration in herbal mixtures and taxonomic evidence.
Materials and Methods
Plant materials
The present study was mainly based on fresh materials collected from natural habitats (University of Sadat City Farm, Egypt) in addition to specimens obtained from well identified herbarium specimens, the voucher specimens are deposited at Environmental Studies and Research Institute Herbarium, University of Sadat City Egypt and Faculty of Science Mansoura University herbarium. The aerial parts of the examined plants were collected during the flowering and fruiting period April 2018.
Anatomical investigations
Samples for anatomy of leaves were chosen from both dry and fresh material. Dried herbarium specimens of leaves were first softened by either normal or warm water, after fixation specimens were transformed in ethyl alcohol, and then embedded in paraffin wax. The specimens were sectioned 10-15um; sections were dehydrated in alcohol-xylol series. Sections were stained in safranin and light green according [18]. The transverse sections of leaves were examined by using light microscope. The trichomes investigated plant followed the terminology after [19,20].
Pollen grain investigations
Collection of pollen grains: Pollen grains were extracted from fresh flowers of plant specimens collected from the Royal Farms for Medicinal plants (Giza Governorate, Egypt) during spring season 2018, and compared with already well identified voucher specimens of the herbarium of Surveys of Natural Resources Department, Environmental Studies and Research Institute, University of Sadat City, Egypt. The studied pollen grains were prepared according to the method of [21].
Acetolysis
The collected pollen grains were acetolysed following the procedure of [21]. Fresh anthers were placed in a centrifuge tube and macerated in 70% ethyl alcohol stirring with a glass rod, this dehydrated the pollen grains, then centrifuged at the speed of 5,000 revolutions per minute for fifteen minutes. The alcohol was poured off, glacial acetic acid was added, and the mixture was centrifuged again at the same speed. Acetolysis mixture (sulphuric acid and acetic anhydride 1:9) was added next and the tube containing the pollen grains and the mixture was kept in water bath and heated from 70 °C to boiling point. The mixture was then stirred with a glass rod and centrifuged again at the same speed as above, after which the acetolysis mixture was decanted and glacial acetic acid added. The mixture was centrifuged again, distilled water added, followed by another centrifugation. Water was then decanted and the acetolysed pollen grains were mounted in dilute glycerin solution for glass slides permanent mount (Figure 1).
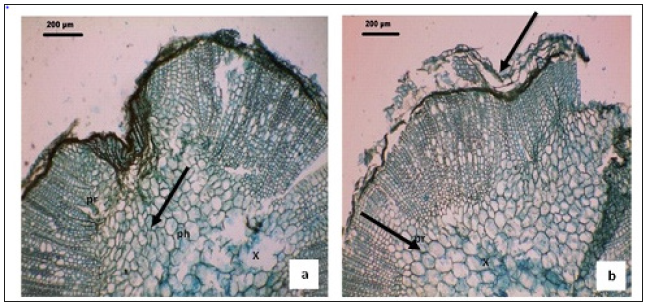
Figure 1:Anatomical characteristics of Ocimum basilicum L. leaves (a & b) Cross-sections of the root of Ocimum basilicum L. pr: pith rays. Bars=200μm.
Light microscopy examination
Acetolysed pollen grains were properly examined under the light microscope according to [22]. Pollen diameter (μm), Pollen wall thickness (μm), Depth of colpi (μm), Distance between colpi (μm), Pore diameter (μm), Number of pores were measured. Also, pollen grains size, Colpi length, pollen shape, tectum surface and colpi type of 20 pollen grains were taken with the aid of an ocular micrometer inserted in the eyepiece of the microscope. These measurements were later multiplied by the ocular constant with respect to the power under which they were taken. Photomicrographs of the acetolyzed pollen grains were taken, while pollen morphological descriptions are based on features recognized at x1000 magnifications and photographs were taken with an Olympus BX21 light microscope while, pollen slides prepared were kept in special slide holders at the herbarium of Surveys of Natural Resources Department, University of Sadat City, Egypt. Pollen grains were mounted on slides in glycerin jelly and photographed.
Pollen ultrastructure investigations
For scanning electron microscopy (SEM), pollen air dried using 95% ethanol was mounted on a glass coverslip attached to an aluminum stub and then coated with gold, to a thickness of 300A, using a Jeol JFC 1100 sputter coater, then viewed at 20KV in a JOEL JSM 5300SEM of the Central Laboratory, Faculty of Agriculture, Mansoura University, Egypt. The subsequent examination was with a Tesla BS 340SEM. Selected SEM micrographs were then digitized and classified using image analysis software. While analysis of variance and Scheffe’s test were used for statistical evaluation [23], results are expressed as mean±standard deviations. The measured polar axis and equatorial diameter were based on at least 25 samples and other characters on approximately 20 under the LM. All of the measurements were done using Caranoy 2 [24]. The U-test was applied using SPSS 10.0. Pollen terminology follows [25] and [26].
Trichomes investigations
Trichomes obtained from fresh plant material were made with the aid of a light microscope. Observations and measurements of the examined identified types of the trichomes followed the terminology according to [21,22].
Results
Pollen grains characteristic features
Pollen types present were of hexacolpate and convex in shape (Figure 2). The mean diameter of the pollen grains was 65.80±0.94 um (Table 1), the apertures were situated in both the polar and equatorial views of the pollen grains. Pollen diameter, the wall thickness recorded 7.61±0.13um, the depth of colpi is 19.98±0.52um, while distance between the colpi 25.78±0.18um, the pore diameter is 7.37±0.59 um and the numbers of pores of the pollen grains 38.88±0.78um are shown in (Table1 and Figure 2 & 3). (Figure 2a & 2b is the polar view – c is the equatorial view), pollen shape: suboblate; Aperture: Stephanocolpate (hexa-zonocolpate); slit shaped, margo present, the sculpture: Pre-reticulate, reticulum continuous all over the surface. The grain size: 65um. Tectum: mega reticulate, (Figure 2a & 2b) and (Figure 3a & 3d). Data obtained from (Table 2, Figure 2a, 2b & 2c), it was clearly light microscopy photographs of pollen grains of the Ocimum basilicum L (a and b) polar view. (c) Equatorial view, while the pollen size about 65um with magnification power: (×=1000). The polar axis was 65.16um while the equatorial one 64.98μm, and the average P/E ratio was 1.01um (Table 2). Tectum examined by SEM showed bi-reticulate patterns and deep with regular rough granular particles of the inner part of substratum and enlargement part exine (Figure 2c & 2d).
Figure 2:ALight microscopy photographs pollen grains of the Ocimum basilicum L, (a and b) polar view. (c) Equatorial view. Magnification power: (x=1000), Scale bars = 10um. Tectum: (mega reticulate, photos a and b)
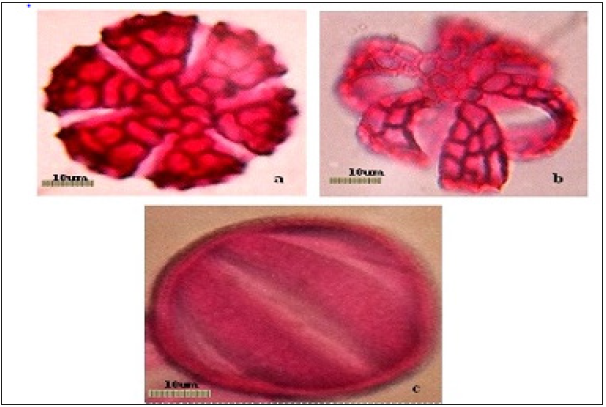
Figure 3:Scanning electron microscope micrographs of pollen grains. (a) Polar view; (b) Equatorial view; (c & d) enlargement part of exine (mega reticulate, black arrow)
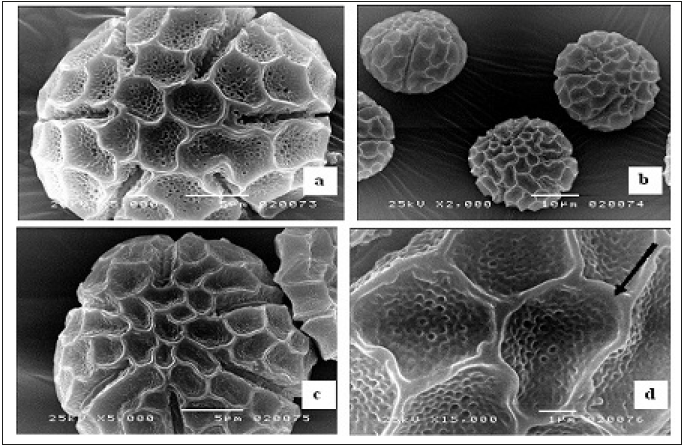
Table 1:
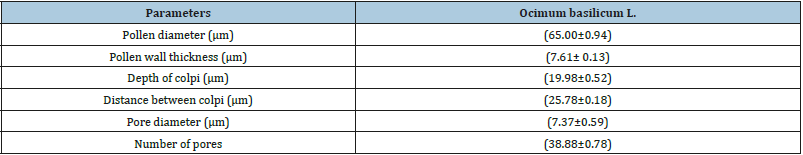
Table 2:

Pollen Description
i. Shape: Suboblate.
ii. Aperture: Stephanocolpate, (hexa-zonocolpate), margo present.
iii. Sculpture: Mega-reticulate, reticulum continuous all over the surface.
iv. Lumina: Granulated
Anatomical characteristics of Ocimum basilicum L. leaves
(Figure 1a&1b): The leaf consists of adaxial concavity and abaxial prominent midrib with the lamina directed towards upper side (Figure 1a). The midrib is somewhat bowl shaped in sectional view (Figure 1b) it is 1.5mm wide and 60um thick. The ground tissue is parenchymatous and the cells are small, polygonal and compact. The vascular strand is single, wide and bowl shaped (Figure 1b), while the vascular strand consists of several short three or four cells long, angular narrow xylem elements with wide parenchymatous gaps in between. The phloem elements located along the lower end of xylem strand and the phloem is seen in small discrete masses.
Microscopical examination of leaf and flower powder
Microscopical examination of leaves and flower powders showed presence of numerous non- glandular trichomes of average length 90um and diacytic type stomata, whereas, T.S of leaf showed that the leaf has midrib and a thin lamina with uneven lower epidermis attached at the lateral sides of the upper side (Figure1a & 1b), also upper and lower epidermis showed simple covering uniseriate non glandular trichomes, as well as sessile short stalked glandular ones (Figure 4a-4d).
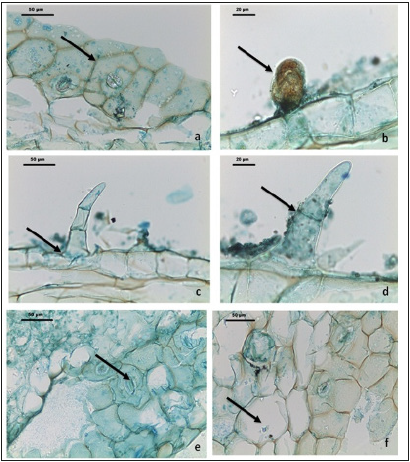
Figure 4:Micro morphological characteristics of Ocimum basilicum L. leaves: (a & d). Diacytic type Stomata (b). Glandular capitate trichomes (c & d), marginal non glandular trichomes (f), Parenchyma cells with ca- oxalate crystals.
Trichome micromorphology
Non-glandular trichomes: Non-glandular trichomes (Figure 4c & 4d): are non-secretory covering type, they occur on the surface or along margins of the lamina. The trichomes are either unicellular or multicellular, uniserate unbranched were observed on different parts of the plant, including the leaves and flowers. The observed trichomes are broader at the base and tapering at the tip, (arrect or bent) with smooth surface wall (Figure 4c & 4d). The glandular trichomes (Figure 2b): Peltate type is seen in the powder of the leaves, few glands containing essential oil are seen attached on the lamina (Figure 2b). The gland is circular plate with a central stalk; at the tip of the stalk occurs unicellular spherical or hemispherical glandular body and darkly stained secretory cells, the gland size measured 60um in diameter of peltate cup-shaped, (Figure 4b).
Discussion
The present study provides useful information on the micromorphology, palynology of pollen grains, leaves anatomy and trichomes morphology of sweet basil (Ocimum basilicum L.) which consider the main of all basil species used as pharmacopeial medicinal plants in Egypt [27]. Data and observations of pollen ultrastructure revealed that, mega reticulated form pollen sculpture and granulated lumina in the studied Ocimum basilicum L. in accordance with those of [9], it was clearly from light microscopy photographs of pollen grains of the Ocimum basilicum L. (Figure 2a & 2b) polar view and (c) Equatorial view, with pollen size about 65um. The polar axis recorded 65.16 um while the equatorial one 64.98 μm, and the average P/E ratio was 1.01um with aperture: Stephanocolpate, (hexa-zonocolpate), six colpi, (Table 2). On the other hand, tectum examined by SEM showed bi-reticulate patterns and deep with regular granular particles of the inner part of substratum and enlargement part exine (Fig. 2c & 2d). In a similar study, [28] used pollen grain sizes to group some species O. basilicum var. purpurascens and O. gratissimum are more closely related based on pollen grain attributes, since they clustered together at a higher similarity level than that of O. basilicum and O. canum. From the main characteristic element founded (mega reticulation sculpture) which more specific to Ocimum basilicum L of all Ocimum genus. The results of the present study revealed that the pollen characters of the Ocimum basilicum L. are valuable in taxonomic applications and may be useful in quality control (microscopic examinations of medicinal plants). [29,30] reported that, aerial parts of the aromatic plants belonging to the family Lamiaceae are covered with trichomes, including non-glandular and glandular or secretory trichomes. In the present study, the results recorded two types of non- glandular and one type of glandular trichomes of the leaves and flowers powder. The non-glandular trichomes are traditionally known as plays an important role in the physical protection of plants against biotic and abiotic stresses, on the other hand, these trichomes acting as a mechanical barrier against, high light intensity and temperatures, and feeding activities of different types of harmful insects. [31] stated that, for the Lamiaceae family, there are different plants species can have different densities of nonglandular and glandular trichomes, and different morphological features which could be of high important taxonomic values. The observed non glandular trichomes with average length 90um are broader at the base and tapering at the tip, (arrect or bent) with smooth surface wall (Figure 4c & 4d). In present study, one distinct glandular trichomes, short-stalked capitates, and peltate glandular trichomes were found (Figure 4b). While, the glandular trichomes (Figure 2b): Peltate type is seen in the powder of the leaves, few glands containing essential oil are seen attached on the lamina, at the tip of the stalk occurs unicellular spherical or hemispherical glandular body and darkly stained secretory cells, the gland size measured 60um in diameter of peltate cup-shaped, (Figure 4b). In this connection, [32] stated that glandular trichomes are the primary sites of secondary metabolite biosynthesis and plays an important role in secretion and storage biological substances and essential oils [33]. The findings of [29] who reported that capitates trichomes are variable in stalk length, glandular head shape and secretions so can be classified into various types. The short-stalked capitates trichomes with size measured 60um in diameter (Figure 4b) are represented in the studied plant Ocimum basillicum L. [34] stated that the short-capitate trichomes are the commonest type of capitates trichome found in Lamiaceae. Previous studies reported that, many species of Lamiaceae family have a broad head of peltate glandular trichomes with four to twelve cells [35,36]. Microscopical observations and anatomy of leaf showed that, the leaf stomata were of the diacytic type (Figure 4e) and the size (29um±2) while the guard cells with two subsidiary cells [37]. Findings of this study revealed that, the stomata of diacytic type and more characteristic of Ocimum basilicum L. leaves they play important role as a potential element as indicators of such aromatic plant [38].
Conclusion
In the present study we can concluded that, O. basilicum L (sweet basil) belonging to the Lamiaceae family known as the ‘King of Herbs’ has been used in traditional medicine. The leaves are rich in essential oil and secondary metabolites of biological and therapeutic importance to cure different diseases. This study is the first of its kind that primarily focuses on the pollen micromorphology and leaves trichomes, stomata of O. basilicum L (sweet basil) in Egypt.
a) The present study demonstrated uneven pollen sculpturing (mega reticulate tectum) of Ocimum basilicum L. (sweet basil).
b) Uneven distribution of glandular, non-glandular trichomes and diacytic stomata on the leaves.
c) We distinguished two types of non-glandular trichomes and one type of glandular trichomes. The short capitate glandular trichomes showed high morphological differentiation, the non-glandular trichomes of these species participate in the chemical interaction of the plants with the environment.
References
- Pooja A, Malathi N, Chamundeeswari D (2014) Pharmacognostic evaluation of leaves of Ocimum basilicum linn, the Lamiaceae Journal of Chemical and Pharmaceutical Sciences 7(3): 250- 253.
- Abhay KV, Rashmi k, Arun KS, Nagaraju B, Mukesh CS (2014) Synthesis, characterization, antimicrobial, analgesic and anti-inflammatory activity of novel mannich bases of benzimidazole derivatives. World journal of pharmaceutical research 3(4) :517-528.
- Bilal A, Jahan N, Ahmed A, Bilal SN, Habib S, etal. (2012) Phytochemical and pharmacological studies on Ocimum basilicum linn: A review. International Journal of Current Research and Review 4(23): 73-83.
- Boulos L (2009) Flora of Egypt checklist-revised annotated edition 2009. Open Journal of Ecology.
- Politeo O, Jukic M, Milos M (2007) Chemical composition and antioxidant capacity of free volatile aglycones from basil (ocimum basilicum l) compared with its essential oil. Food Chemistry 101: 379-385.
- Juliani HR, Simon JE (2002) Antioxidant activity of basil. In: Janick J, Whipkey A (Eds.), Trends in new crops and new uses. American society for horticultural science press, Alexandria, USA, pp. 575-579.
- Shafique M, Khan SJ, Khan NH (2011) Study of antioxidant and antimicrobial activity of sweet basil (ocimum basilicum) essential oil. Pharmacology online 1(1):105-111.
- Erdtman G (1945) Pollen morphology and plant taxonomy. IV. Labiatae, Verbenaceae and Avicenniaceae. Svensk Botanisk Tidskrift 39: 279-285.
- Harley MM, Paton A, Harley RM, Cade PG (1992) Pollen morphological studies in tribe Ocimeae (Nepetoideae: Labiatae): I. Ocimum L. Grana 31(3): 161-176.
- Abu-Asab MS, Cantıno PD (1994) Systematic implications of pollen morphology in subfamilies Lamioideae and Pogostemonoideae (Labiatae). Annals of the Missouri Botanical Garden 81(4): 653–686.
- Moore PD, Webb JA (1978) An illustrated guide to pollen analysis. Journal of Archaeological Science p.133.
- Arogundade OO, Adedeji O (2009) Pollen grain morphology of three species and a variety of Ocimum Linn (Lamiaceae) in southwestern Nigeria. Journal of Science and Technology 29(3): 1-7.
- Gupta VK, Singh J, Kumar R, Bhanot A (2011) pharmacognostic and preliminary phytochemical study of ocimum gratissimum linn (family: lamiaceae). Asian Journal of Plant Sciences 10(7): 365-369.
- Cantino PD, Harley RM, Wagstaff SJ (1992) Genera of labiatae: status and classification. In: Harley RM, Reynolds T (Eds.) Advances in Labiate science. Royal Botanic Gardens, Kew, London, UK, pp. 511-522.
- Cantino PD (1990) The phylogenetic significance of stomata and trichomes in the labiatae and verbenaceae. Journal of the Arnold Arboretum 71(3): pp. 323-370.
- Harley RM, Atkins S, Budantsev AL, Cantino PD, Conn BJ, et al. (2004) Labiatae. In: Kadereit JW (Ed.) The families and genera of vascular plants, Lamiales. Springer, Berlin, Germany, 7: 167-282.
- Navarro T, El Oualidi J (2000) Trichome morphology in Teucrium L. (labiatae): A taxonomic review. A Jard Bot Madrid 57: 277-297.
- Sass JE (1961) Botanical microtechnique (3rd edn). In: Sass JE (Ed.) Botanical microtechnique. The low state University Press, Ames, USA.
- Payne W (1978) A glossary of plant hair terminology. Brittonia 30(2): 239-255.
- Giulian C, Malcibini L (2008) Insight into the structure and chemistry of glandular trichomes of Labiatae, with emphasis on subfamily Lamioideae. Plant Systematics 276: 199- 208.
- Erdtman G (1960) The acetolysis method-A revised description. Svensk Botanisk Tidskrift 54: 561-564.
- Moore PD, Webb JA, Collinson ME (1991) Pollen analysis. Blackwell Science edition.
- Sokal RR, Rohlf FJ (1981) Biometry. WH Freeman and Company, San Francisco, California.
- Schols P, Dessein S, Hondt C, Huysmans S, Smets E, et al. (2002). A new digital measurement tool for palynology. 41: 124-126.
- Faegri K, Iversen J (1975) Textbook of pollen analysis. Munksgaard, Copenhagen, Denmark.
- Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, et al. (2009) Pollen terminology. New York, USA.
- Egyptian Pharmacopoeia (1984) General organization for governmental printing office, Cairo, Egypt.
- Akinwusi O, Illoh HC (1996) Pollen grain morphology of some species of Hibiscus Linn: Nigerian Journal of Botany. 9: 9-14.
- Werker E, Ravid U, Putievsky E (1985) Structure of glandular hairs and identification of main components of their secreted material in some species of Labiatae. Israel Journal of Botany 34(1): 31-45.
- Werker E (1993) Function of essential oil-secreting glandular hairs in aromatic plants of the lamiaceae- a review. Flavour Fragrance Journal 8(5): 249-255.
- El-Gazzar A, Watson IA (1970) A taxonomic study of labiatae and related genera. New Phytol 69: 451-486.
- Weiss EA (1997) Rosaceae. In: Essential Oil Crops, CAB International Wallingford, Oxon, UK, 393-416.
- Clark LJ, Hamilton JGC, Chapman JV, Rhodes MJC, Hallahan DL (1997) Analysis of monoterpenoids in glandular trichomes of catmint Nepeta racemose. Plant Journal 11(6): 1387-1393.
- Ascensa LO, Mota L, Castro M (1999) Glandular trichomes on the leaves and flowers of Plectranthus ornatus: morphology, distribution and histochemistry. Annals of Botany 84(4): 437- 447.
- Bagherpour S, Kahraman A, Dogan M, Celep F, Baser B, et al. (2010) The anatomical and micromorphological characteristics of Salvia vermifolia (Section Aethiopis Bentham, Lamiaceae) from Central Anatolia. Central European Journal Biology 5(6): 872–879.
- Kahraman A, Celep F, Dogan M (2010) Anatomy, trichome morphology and palynology of Salvia chrysophylla Stapf (Lamiaceae). South African Journal of Botany 76: 187-195.
- Mauseth JD (1988) Plant anatomy. The Benjamin/Cummings Publishing Company, Inc., Menlo Park, California, USA.
- Davis AR, Gunning BES (1992) The modified stomata of the floral nectary of Vicia faba L. Protoplasma 166: 134-152.
© 2019 Azzazy MF. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






