- Submissions

Full Text
Advances in Complementary & Alternative medicine
Synthesis of Hydroxyapatite 2-Hydroxyethyl Methacrylate Blended Material for Dentistry
HKGKDK Hapuhinna1, RD Gunaratne1* and HMJC Pitawala2
1 Department of Engineering Technology, Sri Lanka
2 Department of Science & Technology, Sri Lanka
*Corresponding author: RD Gunaratne, Department of Engineering Technology, University of Sri Jayewardenepura, Nugegoda, Sri Lanka
Submission: June 27, 2019;Published: July 15, 2019

ISSN: 2637-7802 Volume5 Issue1
Abstract
As a bio ceramic hydroxyapatite and its composites have applied in several biomedical applications. This research used to examine suitability of Hydroxyapatite 2-hydroxyethyl methacrylate composite for dentistry. Sri Lankan chlorapatite, ethanol and dil. acid used to synthesize hydroxyapatite by sol gel technique and then it was reinforced with polymer prior to compare and contrast it’s chemical and physical properties with human tooth and commercial dental filling material. Results show there is a close similarity between newly synthesized product and human tooth. It has credent high thermal stability, good crystalline and porous properties than the commercial product. At the end, the study concluded that the new material can be used directly as a substitution for human tooth.
Keywords: Hydroxyapatite; Dental fillings; 2-hydroxyethyl methacrylate; Dentistry; Human tooth
Introduction
Hydroxyapatite is a widely used bio ceramic which has a close chemical and structural similarity with human hard tissues and performs several outstanding properties biocompatibility, non- inflammatory in nature, osteoconductive, non-toxicity, bioactivity etc. [1-11]. As a result, it has a range of biomedical applications mainly in the fields of orthopedics and dentistry [12-25].
Eppawala hydroxyapatite is synthesized using sol gel technique under alcoholic route as a value addition to Sri Lankan Eppawala chloroapatite, as it is only considered as a raw material for fertilizer industry up to the date [21,25].
This study mainly focused to find out suitability of Eppawala Sol Gel Synthesized Hydroxyapatite (SGHAp) as a dental filling material, by comparing and contrasting it with human tooth as well as commercially available Glass Ionomer Cement (GIC), which is currently used for dental applications in Sri Lanka. Selected commercial product containing two main parts as GIC majorly consists of Fluor aluminosilicate glass and 2-hydroxyethyl methacrylate polymer, is currently used self-curing conventional glass ionomer restorative material in Sri Lankan government hospitals which indicates for non-stress bearing restorations, deciduous teeth restorations, geriatric restorations, intermediate restorative and base material for cavities using sandwich technique, cervical restorations, cold build ups, temporary fillings and dentine replacement etc. It is prepared directly before use by mixing its powder component with polymer component clinically and resulted paste is cured within a few seconds [26].
When consider 2-hydroxyethyl methacrylate polymer it is a hydroxyester and a monomer resin widely used as a desensitizing agent for dentistry applications, as its stimuli pain by get sealing into sensitive areas in the teeth and blocking the dentinal tubules at the dentin surface [27]. As this study designed only to find out the possibility of substituting our newly synthesized ceramic powder into GIC powder of commercial product, we are only focusing to find out structural suitability of ceramic product as dental filling material.
Sample preparation
As the first step, natural raw apatite mineral was collected from the Eppawala apatite site. Then they were sorted as High-Grade Rock Phosphate (HERP) by the visual appearance of less coated apatite. After removing mud, collection of apatite rocks were dried under sunlight, crushed using a jaw crusher (Serial no: 1720011, China) into small crystals /powder, grind further into micron/nano level HERP powder using a planetary ball mill (XQM-4.0A) and sieved using sieve set (A060_01AC/0219, Scotland). Less than 63-micron range particle size powder were collected, and oven dried at a temperature around 120 °C for 5hrs to prepare moisture removed HERP powder (MHERP).
A Sol Gel Synthesized Eppawala Hydroxyapatite (SGHAp) was prepared under sol gel technique acidified route using MHERP, absolute ethanol, and diluted acid as the raw materials. Mixture of MHERP and dil. acid (1:1 ratio) was stirred well in absolute ethanol medium for 4-6hrs until the formation of gel. Then it was oven dried to a temperature less than 120 °C for 15hrs and again two stage heat sintering were done starting from 400 °C to 750 °C for 8hrs [2,22]. Finally, the synthesized ceramic powder was mixed with commercial 2-hydroxyethyl methacrylate polymer until a paste form. Commercial GIC powder and 2-hydroxyethyl methacrylate were mixed together until a paste forms, that sample was used to compare synthesized ceramic composite.
Sample characterization & analysis
Before mixing with the polymer, commercial GIC powder and Eppawala hydroxyapatite was examined under X-ray Fluorescence Spectroscopy (Rigaku XRF spectrometer) to find out its elementary composition and presence of impurities. The polymer was identified using Fourier Transform Infrared Spectroscopy (Bruker-Alpha FTIR spectroscopy) under ATR technique. Then the mixtures of newly synthesized product and commercial product along with the human bone were characterized using XRD, FTIR, TGA, and SEM with EDS techniques. The crystallographic phases of samples were determined by X- ray diffractometer (Rigaku-Ultima. IV diffractometer) in reflection mode with Cu Kα1: 0.154nm radiation.1.5O min-1 scanned speed was used to collect data within 2θ angles range from 15° to 80°. The presence of functional groups was confirmed by FTIR over the region 400-4000cm-1 using KBr pellet technique. The resolution of the spectrometer was 4cm-1. The surface morphology and microstructural features of samples were studied using Hitachi SU6600 Analytical Variable Pressure FE-SEM (Field Emission Scanning Electron Microscope) and Oxford Instruments EDX with AZtec software. Furthermore, Thermogravimetric analysis (TGA) was done using a Thermal Analyzer (SDT Q600) with N environment, 10 °Cmin-1 heating rate, and 1450 °C maximum temperature to find out the thermal stability of samples.
Results and Discussion
Sol gel synthesized Eppawala hydroxyapatite (SGHAp) powder contains Ca, P and O include in higher weight percentages and Fe, Al and Si as the impurities with hexagonal crystal structure showing a close similarity with mammalian bones and consists of many correlated, needle shape crystalline particles while credenting good thermal stability [2,22]. Results for SEM with EDS Analysis of sol gel product with polymer mixture, commercial GIC with polymer mixture and human tooth are given in the Tables 1-3.
Table 1:Shows that sample contains Al and Sr in higher amounts and S, Fe, Ca and Ba in less amounts [26].
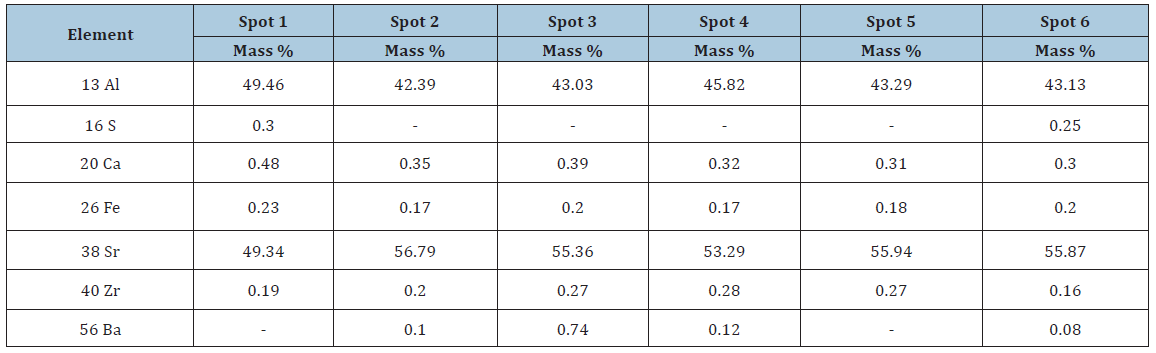
Table 2:SEM with EDS results for sol gel product with polymer mixture, commercial GIC with polymer mixture and human tooth.
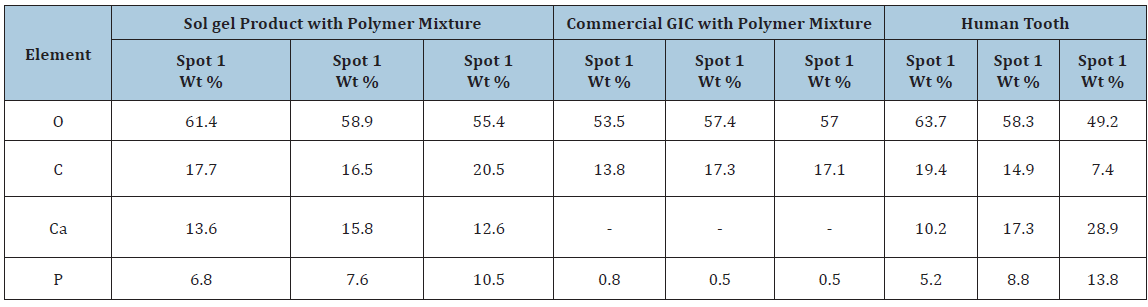
Table 3:
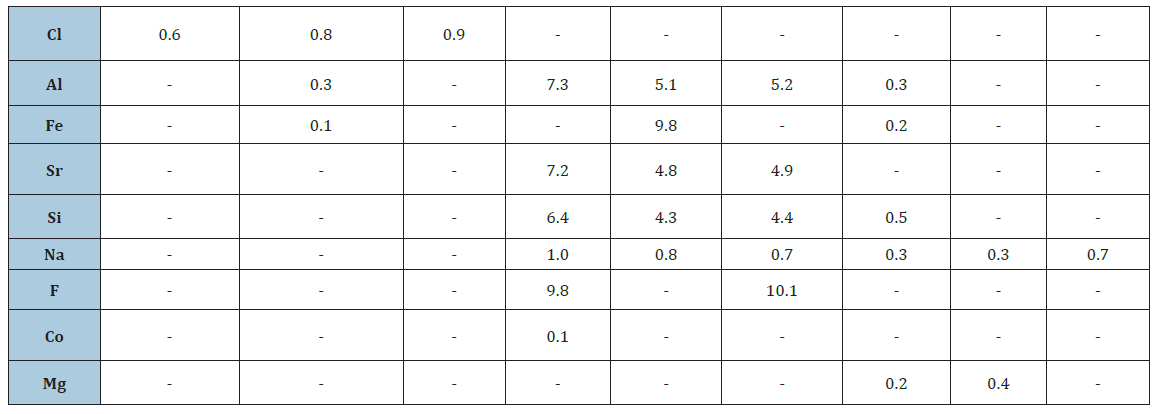
According to that; SEM with EDS images of SGHAp with polymer mixture show that sample contains O, Ca in higher amounts and then P, C, Cl in order. Fe also found in very less amount as an impurity. Commercial product mixture contains O, C presence in higher amounts of the products and in order F, Al, Sr, Si, N, P and negligible amount of Ca. Human tooth contains O presence as the highest amount and then C, Ca, P and Si in order. Na, Al, Fe, Mg presence in very fewer amounts. When comparing results, it can be stated that a human tooth and the mixture of SGHAp with polymer have similarity in composition.
Considering Figures 1-3; SEM images of all mixtures and human tooth show that there are good correlations of particles. SGHAp with polymer and human tooth consist of crystalline particles. SGHAp mixture specially carried highly crystalline needle shaped particles.
Figure 1:
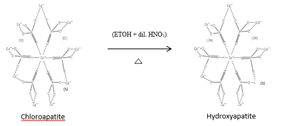
Figure 2: SEM images for SGHAp with polymer mixture, 10.0kv,

a) 10K
b) 5KX
c) 2KX
d) 500X
Figure 3:SEM images for commercial GIC mixture, 10.0kv.

Figure 4 shows that the resulted graph for polymer it has coincided with the FTIR characteristic graph for 2-hydroxyethyl methacrylate, therefore polymer in the commercial product was confirmed as the 2-hydroxyethyl methacrylate polymer. It interprets several peaks related to stretching vibrations including a sharp intense peak at 1731cm-1 related to the presence of ester carbonyl group, broad peak nearly 1150cm-1 due to the C-O (ester bond) and a peak nearly 1250cm-1 is due to the vibrations of C-C bond. Also, literature shows the broad peak ranging from (3000-3500) cm-1 is owing to the presence of stretching vibration [27]. As shown in the Figure 5, all peaks
e) 10K
f) 5KX
g) 2. 5KX
h) 500X
Figure 4:SEM images for human tooth, 10.0kv.

i) 30K
j) 5KX
k) 2KX
l) 500X
Figure 5:FTIR graph for polymer.
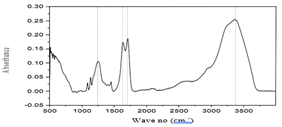
Figure 6:FTIR comparison for commercial GIC with polymer mixture, SGHAp with polymer mixture, and human tooth.
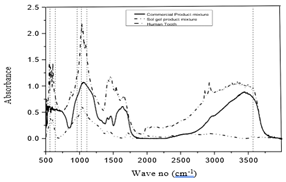
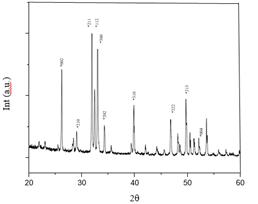
Figure 7:XRD pattern for SSHAp with polymer mixture.
for phosphate groups in the 560cm-1, 640cm-1, 963cm-1, 1028cm-1 and 1110cm-1 wave no range and characteristic peak for OH-/ hydroxyapatite nearly 3572cm-1 waves no appeared in the human tooth as well as the SGHAp with polymer mixture [2,22]. It confirms that even after the mixing, the presence of hydroxyapatite in the SGHAp product. When considering commercial product mixture, it shows peak nearly 3572cm-1 wave no range, which may due to the presence of OH- group, but that couldn’t be identified as hydroxyapatite characteristic peak, as no peaks found related to phosphate groups. Apart from that, some peaks can be found commonly in both commercial product mixture and SGHAp mixture except in human tooth nearly (750cm-1- 2000cm-1) wave no range and 3000cm-1 wave no range which is also appeared in Figure 4, they are the related peaks for 2-hydroxyethyl methacrylate. As a result, it can be concluded that both commercial product and SGHAp mixed well with the polymer.
Figure 6 explains even after mixing 2-hydroxyethyl methacrylate polymer, XRD results of SGHAp mixture all characteristic peaks related to the crystallographic phases 002, 210, 211, 112, 300, 202, 310, 222, 213 and 004 of hexagonal hydroxyapatite, which shows similarity to human tooth [2,22,28,29]. Comparing those results with Figure 7, XRD pattern for commercial GIC with polymer it has carried out an amorphous structure without crystalline properties.
13.1860mg of SGHAp with polymer mixture was subjected to TGA as shown in the Figure 8 First significant weight loss which occurs in between 0 0C-320 °C (0.2705mg) representing 2.051%, may associate with the dehydration of the sample. Then again up to 625 °C, 0.1691mg weight loss indicating 1.282% was occurred and following that interval the sample reduced its weight nearly 0.7776%, 0.1025mg at 903 °C; they may occur due to the gas elimination. Then again from 903 °C up to 1431.45 °C there is a weight loss indicating 0.6794% (0.0895mg) which has occurred due to the incipient transformation of produced hydroxyapatite in β-TCP. Therefore, it indicates the formation of hydroxyapatite in products. At 1431.45 °C 95.21% of the original weight was remained.
Figure 8:XRD pattern for commercial GIC with polymer mixture.

As figured in Figure 9, 14.7280mg commercial product mixture was subjected to TGA. First significant weight loss which occurs in between 0 0C-250 °C (1.626mg) representing 11.04%, may associate with the dehydration of the sample. Then again up to 500 °C, 1.628mg weight loss indicating 11.06% was taken place, it may occur due to the gas elimination. Following that, some weight losses occur in between small intervals indicating 7.019% of initial weight (1.034mg) 765 °C to nearly 950 °C, 1.342% of initial weight (0.1977mg) 950 °C to 1200 °C those may occur due to the structural deformation and elimination of some gaseous compounds with different molecular weights. At 1434.27 °C 69.60% of the original weight has remained.
Figure 9:TGA curve for SSHAP with polymer mixture.
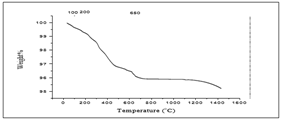
According to the Figure 10, 14.3800mg human tooth sample was subjected to TGA. As mentioned in the literature; first significant weight loss which occurs nearly at 200 °C (0.9166mg) representing 6.374%, may associate with the dehydration of the sample. Following that interval, the sample reduced its weight nearly 3.378mg at 650 °C; it has occurred due to the tooth structure collagen elimination. This reaction continues up to 1100 °C, with a lowered rate. Above that temperature, a fine TGA curve descending slope is observed up to maximum analyzed temperature of 1436.52 °C with the total weight loss of 28.60%, this being associated with the collagen remains removal & the incipient transformation of hydroxyapatite in β-TCP [2,22,28,30].
Figure 10:TGA curve for commercial GIC with polymer mixture.
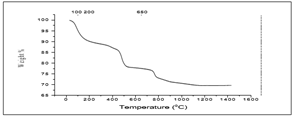
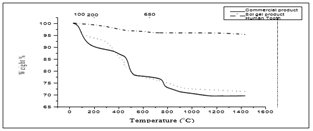
When comparing human tooth with commercial GIC with polymer mixture and SGHAp with polymer mixture, according to Figure 11 & 12, all three samples have shown relatively same pattern of weight loss. But due to the composition similarity, weigh loss pattern has a similarity 650 °C to 1430 °C temperature range of human tooth and SGHAp with polymer mixture sample than commercial product as they were containing hydroxyapatite. Also due to the least amount of weight loss in synthesized SGHAp mixture sample than tooth and commercial product mixture, it can be concluded that the synthesized SGHAP with polymer mixture perform high thermal stability and good material stability in nature and application.
Figure 11:TGA curve for human tooth.
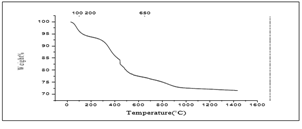
Figure 12:Comparison between TGA results of human tooth, commercial product mixture, and sol gel product mixture.
Conclusion
Study concludes that sol gel synthesized hydroxyapatite ceramic embedded 2-hydroxyethyl methacrylate mixture can be used as a direct substitution for dental filling material as it performs chemical and structural similarity with human tooth as well as interprets highly crystalline structure and good thermal stability.
References
2. ME (2011) Hydroxyapatite-Based Materials: Synthesis and Characterization. Biomedical Engineering-Frontiers and Challenges.
6. Zobnenovice (2017) Hydroxyapatite: properties, uses and applications. FLUIDINOVA.
10. Case study: Polymer matrix composites in automobiles.
11. Innovative materials for innovative automobiles.
16. Composite manufacturing.
20. Hapuhinna H, Gunaratne R, Pitawala H (2019) A composite developed from a methyl methacrylate and embedded Eppawala hydroxyapatite for orthopedics'. World Academy of Science, Engineering and Technology, International Journal of Materials and Metallurgical Engineering 13(6): 35- 44.
21. Anon Eppawala rock phosphate deposit and processing plant. Wicky's Blog.
22. Hapuhinna H, Gunaratne R, Pitawala H (2018) Development of a biomaterial from naturally occurring chloroapatite mineral for biomedical applications. World Academy of Science, Engineering and Technology, International Journal of Materials and Metallurgical Engineering 12(8): 380-388.
23. Hapuhinna H, Gunaratne R, Pitawala H, Wijesekara K, Ekanayake E (2017) Synthesis and characterization of hydroxyapatite from Eppawala Rock Phosphate for Biomedical Applications as a value-added product.
24. Anon, Industries from Eppawala phosphate deposit. Online edition of Daily News - Features.
25. Ratnayake SP, Navaratna AN. Spectroscopic determination of metal impurities in commercial raw material fertilizer of Sri Lanka.
26. (2018) Riva self-cure / riva self-cure HV.
27. Pubchem (2005) 2-Hydroxyethyl methacrylate.
28.
30. Anon (2018) Complex analysis on heat treated human compact bones.
© 2019 RD Gunaratne. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






