- Submissions

Full Text
Advances in Complementary & Alternative medicine
Anti-Influenza Nutraceuticals: Antiviral and Anti-Inflammatory Effects
Brayden Humpherys* and David D Busath, MD
Department of Physiology and Developmental Biology, Brigham Young University, Provo, UT, USA
*Corresponding author:Department of Physiology and Developmental Biology, Brigham Young University, Provo, UT, USA
Submission: May 20, 2019Published: May 28, 2019

ISSN: 2637-7802 Volume4 Issue2
Abstract
Influenza viruses cause upper-respiratory infections in their hosts with symptoms such as severe lassitude, headache, chills, muscle aches and delayed mild cough with signs of fever. These systemic symptoms are due to the release of cytokines by the bronchial epithelial and macrophage cells. However, influenza can cause more than just unpleasant symptoms - it can also be fatal through pneumonia superinfection or extreme cytokine reactions. There are several different methods of inhibiting influenza from entering or being effective inside the bronchial epithelial cells. The most commonly used drug is a Neuraminidase (NA) inhibitor called, oseltamivir, which prevents newly formed viruses from finalizing their budding from an infected cell. There are also several herbs and nutraceuticals that inhibit influenza viruses through this mechanism and others in addition to decreasing the cytokines being produced by the cells. The cytokine inhibition is often through block of the NF-κB pathway. This paper will discuss the anti-influenza and anti-inflammatory effects of eight of these nutraceuticals, Black Currant, Jamaican Sorrel, bee pollen, Echinacea Purpurea, Siberian Ginseng, honey, bee propolis, and Goldenseal. It will also discuss the anti-influenza and anti-inflammatory effects of four often recurring components of these nutraceuticals, luteolin, apigenin, quercetin, and chlorogenic acid.
Keywords:Black currant; Jamaican sorrel; Bee pollen; Echinaforce; Siberian ginseng; Honey; Bee propolis and goldenseal; Mechanism of action; NF-κB; Hibiscus sabdariffa
Introduction
Influenza is an upper respiratory infection that causes mild to severe symptoms, sometimes resulting in hospital visits and even death. There are often other complications related to influenza as well, such as pneumonia, ear and sinus infections; plus, influenza can augment preexisting chronic medical conditions [1]. The CDC estimates that since 2010 there have been 9.3-49 million cases of influenza in the United States (USA) annually. Of these cases, 140,000-960,000 resulted in hospitalizations, creating a heavy burden on our health care system. In addition, the CDC estimates that around 12,000-79,000 deaths have been caused annually in the USA since 2010 by influenza [2].
A recent review of the influenza’s mechanism of viral infection details how influenza infects its host. It enters through the lungs, binding to sialic acid receptors on the surface of the bronchial epithelia. This binding causes lipid-raft clustering, the activation of Epidermal Growth Factor Receptor (EGFR), a tyrosine kinase that assists with clathrin-mediated endocytosis [3]. Recent studies show that influenza virus binding also can stimulate endocytosis through activation of Platelet Derived Growth Factor Receptor β (PDGFRβ), which stimulates uptake of the virus into endosomes from GM3-rich rafts [4]. After endosomal acidification, the viral M2 channel opens, which allows protons to enter the endosome and weaken the viral core by weakening the protein interactions in the core. By the late endosome, the Hemagglutinin (HA) on the viral envelope undergoes an irreversible conformational change due to the low pH. This causes the viral envelope to fuse with the membrane of the endosome and release of viral ribonucleoproteins (vRNPs) into the cytoplasm. The vRNPs are imported to the cell’s nucleus for transcription of the viral RNA (vRNA), which allows more viruses to be produced and released by the cell [5]. Neuraminidase (NA) which cleaves sialic acid from the cell’s surface, allowing the new virion to be released from the cell. NA also helps the virus move through the mucus to the bronchial cells in the respiratory tract [6].
While influenza is in the endosome, it activates Toll-Like Receptor (TLR) 3 and TLR7, activation of TLR7 causes activation of Myeloid Differentiation primary response 88 (MyD88) which then activates Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB), a transcription factor for many various cytokines. These cytokines are signaling molecules used to alert other cells of the virus. They can have both antiviral and pro-viral effects. It is important to note that in the context of influenza, when NF-κB gets activated and releases cytokines it is often referred to as a “Cytokine Storm” due to the intense systemic inflammation they can cause. Of particular note are the pro-cytokines, Interleukin (IL) 8 and pro-IL-18, which are transcribed through the activation of MyD88. When influenza enters the cell it also activates the Nucleotide binding domain and Leucine-rich Repeat containing Protein 3 (NLRP3) inflammasome which then causes the transcription of capsase-1 which converts pro-IL-1 and pro-IL-18 into their active form (caspase-1 can also activate caspase-3 to induce apoptosis). The newly formed IL-1 can then bind to its receptor, IL-1R, and induce inflammatory gene production through MyD88 signaling. In addition, once the influenza vRNPs enter the cytosol, Retinoic Acid-Inducible Gene 1 (RIG-1) gets activated which can stimulate NF-κB as well [7-11].
Of the cytokines released, IL-6, and Tumor Necrosis Factor-α (TNF-) are particularly pathogenic. The Janus Kinase/Signal Transducer and Activator of Transcription proteins (JAK/STAT) pathway is also important in the cellular response to influenza [12]. When this pathway is activated, various antiviral proteins such as Myxovirus Resistance Protein A (MxA) are transcribed [13,14].
Often NA inhibitors like oseltamivir or zanamivir are used as an early treatment for influenza virus infections. However, due to the high rate of mutation in influenza, some strains have become resistant, or at least less sensitive to oseltamivir. This was particularly evident during the winter 2008-2009 season with H1N1. Unfortunately, NA inhibitors can also have negative side effects, like nausea, vomiting, and psychiatric effects [15].
Fortunately, there are many plants and nutraceuticals such as Jamaican sorrel, honey, bee pollen, bee propolis, Black Currant Berries, Echinacea purpurea, and Goldenseal that have molecular components that have both anti-influenza and anti-inflammatory properties. This is accomplished through blocking NA, HA, NF-B, and other proteins and pathways involved in the influenza infection. Several recent reviews discuss the use of polyphenols and flavonoids for influenza virus infections [16-22]. The purpose of this review will be to discuss the recently discovered mechanisms for the anti-influenza effects as well as the less-appreciated anti-inflammatory properties of the above-named nutraceuticals. While there is a variety of known and unknown compounds in plants effective against influenza, four classes often reoccur in several different nutraceuticals: three structurally related flavones, luteolin, apigenin, and quercetin, and chlorogenic acid with its numerous variants. These compounds will be addressed first followed by a discussion of the eight natural products, many of which are rich in the above compounds. These products have been selected due to their particular effectiveness towards inhibiting influenza based on the literature. The studies used were chosen for their detail in describing the inhibition of the various compounds or product towards influenza.
Recurring components of nutraceuticals
While there are a variety of known and unknown compounds in plants that are effective against influenza, four often reoccur in several different nutraceuticals. Therefore, to avoid unnecessary repetition, these four-luteolin, apigenin, quercetin, and chlorogenic acid- will be addressed separately here. When they occur in a given nutraceutical, they will be mentioned as being present, but their effects won’t be further elaborated.
Luteolin:The flavone luteolin (Figure 1), 2-(3,4-dihydroxyphenyl)-5,7-diihydroxychromen-4-one has been shown to possess anti-influenza properties via block of NA. Lee et al. [22], Liu et al. [23], and Sithisarn et al. [24] found that it inhibited various strains of the influenza virus (Table 1). Lee et al. [22] used a fluorometric method to determine luteolin’s ability to inhibit the viral NA in Madin-Darby Canine Kidney cells (MDCK) cells. Liu et al. [23] used a standard fluorometric assay to determine the amount of inhibition luteolin has against NA. In each case, µM efficacy is observed. Sithisarn et al. [24] used an NA inhibition assay to test luteolin’s ability to inhibit an H5N1 viral NA in A549 human bronchial cells.
Figure 1:Luteolin.
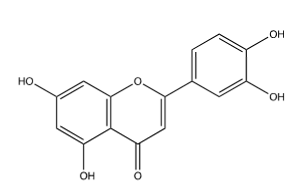
Table 1:The Luteolin effect on influenza.

1 Lee et al. (22);
2 Liu A. et al. (23);
3 Sithisarn et al. (24)
Jia et al. [25] and Pratheeshkumar et al. [26] found that luteolin can also inhibit influenza-induced inflammatory pathways. Pratheeshkumar et al. [26] used human Bronchial Epithelial cells (BEAS-2B), and mice to determine the effect of luteolin on inflammation when hexavalent chromium (an inflammation promoter) was exposed to the cells, and then luteolin was added. They found that luteolin decreased TNF-α expression of MCP-1, VCAM-1, NF-κB, IκBa, and IκB kinase B in a dose dependent manner with 1-2μM concentrations. Jia et al. [25] used an NF-κB transcriptional activity assay as well as immunoblotting analysis of IκB-α in male C57BL/6 mice to reduce the expression of AP-1, HIF-1α, COX-2, iNOS, IL-1β, IL-6, IL-8, MAPK, STAT-3, and TNF-α.
Apigenin:The structures of luteolin and apigenin (Figure 2, 2-(4-hydroxyphenyl)-5,7-diihydroxychromen-4-one) are very similar; in apigenin the catechol (3,4-dihydroxyphenyl) is converted to phenol, lacking the meta alcohol group. They have similar properties in regard to inhibiting influenza and inflammation (Table 2). Liu et al. [23] used the same procedure as with luteolin to find apigenin’s inhibitory effect against NA. Sithisarn et al. [24] also used the same procedures to test apigenin as they did luteolin. Again, the efficacy is significant in the µM range. Apigenin also has been found to inhibit several components of inflammation. Basios et al. [27] found that apigenin inhibited IL-6, MPO, and TNF-α in male Wistar rats after they had had a bilio-pancreatic duct ligation. Palacz et al. [28] found that apigenin also inhibited COX-2, IL-2β, IL-10, TNF-α, and NF-κB expression in RAW-264.7 cells infected with lipopolysaccharide (LPS).
Figure 2:Apigenin.
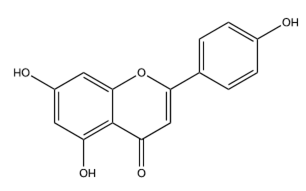
Table 2:The Apigenin effect on influenza.

1 Liu A. et al. (23);
2 2Sithisarn et. al. (24)
Quercetin (Figure 3, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one) is also similar to luteolin, having an added hydroxyl at position 3 on the benzopyran. Wu et al. [29] used cytopathic effect (CPE) assays, Western Blots, immunofluorescence microscopy, and HA binding assays to determine that quercetin inhibits the membrane fusion process of influenza’s entry into the cell by inhibiting the H2 subunit of the HA. Their results for inhibition of influenza infection in cell culture (Table 3) show strong efficacy for three strains. Lee et al. [22] used a fluorometric method as they did when they tested luteolin and found quercetin-3-sophoroside (Figure 4) extracted from Korean Papaver rhoeas bee pollen to be modestly effective towards inhibiting NA. Choi et al. [30] used CPE to demonstrate that quercetin-3-rhamnoside (Figure 5) inhibits A/WS/33 better than does oseltamivir-phosphate at comparable concentrations (Table 4). It should be noted that oseltamivir-phosphate, the prodrug, has been shown to be 100-fold less potent than the active metabolite, oseltamivir carboxylate, in MDCK cell CPE assays [31], but the activity of the quercetin analog is remarkable, nonetheless. For neither compound, did presoaking the virus sample in the compound produce the anti-viral effect, suggesting a post-endocytosis mechanism inhibition. Gel electrophoresis also demonstrated that, like oseltamivir-phosphate, quercetin-3-rhamnoside effectively inhibits the production of viral M segment RNA [30].
Figure 3:Quercetin.
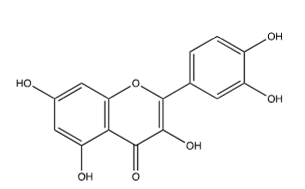
Figure 4:Quercetin-3-Sophoroside.
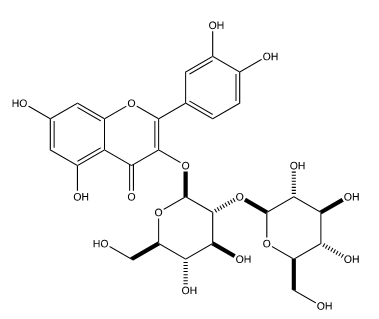
Figure 5:Quercetin-3-Rhamnoside.

Table 3:The Quercetin and Quercetin-3-sophoroside effects on influenza.

1 Wu et al. (22);
2 Lee et al. (29)
Table 4:The Quercetin-3-Rhamnoside effects on influenza1

1 Choi et al. (30)
Takashima et al. [32] found that quercetin moderated the inflammation caused when mice had LPS administered to their trachea by inhibiting TNF-, IL-1, IL-6, and MMP-9. They also discovered that quercetin blocked TNF-, IL-1, IL-6 stimulated by LPS administration in mouse alveolar macrophage cell line AMJ2-C11. Maturu et al. [33] found that inflammation caused by hyperoxia in newborn mice could be relieved through the administration of quercetin. This was in part through the down regulation of NF-B in lung and liver tissues as well as an upregulation in CYP1A1/CYP1B1/NQO1 mRNA. These results suggest that quercetin might inhibit the cytokine storm in influenza infections.
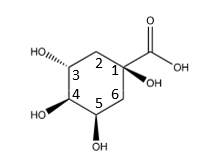
Chlorogenic acid (Figure 6) is a thermally unstable ester that readily dissociates into its components, quinic acid (the tetrahydroxy-cyclohexanecarboxylic acid) and caffeic acid (Figure 7). It and several of its variants are found in several different plants, leaves, roots, and fruits. Green coffee beans, for instance, are a very rich source of chlorogenic acids [34]. Sinisi et al. [35] tested eight different compounds occurring in coffee from roasted green coffee beans that are variants of chlorogenic acid on various viruses such as influenza. The results from the two that were the most effective are in Table 5. Note that the two “R” groups in 3,4-O-dicaffeoyl-1,5-γ-quinide (Figure 8) both refer to ester linkages to caffeic acid groups. The mechanism of action for this chlorogenic acid was inhibition of influenza RNA synthesis, specifically through inhibiting the influenza virus RNA polymerase acidic (PA). It appears that the caffeic acid is the effective component, as it is equally effective alone. It may be advantageous to combine caffeic acid with one of the previous compounds that target sialic-acid binding sites to achieve synergy by blocking at multiple sites.
Figure 6:Chlorogenic acid.

Table 5:The Quercetin-3-Rhamnoside effects on influenza1
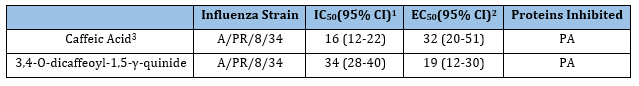
1 Inhibition of endonuclease activity by the PA N-terminus
2 vRNP reconstitution luciferase-based assay in human embryonic kidney (HEK293T) cells;
3 Sinisi et al. [35].
Lin et al. [36] tested nine naturally occurring chlorogenic acid derivatives, in which caffeic acid(s) (Figure 7) was (were) esterized to different hydroxyls on quinic acid (Figure 9). It was found that five of them measurably inhibited viral RNA polymerase (Table 6), with the 4,5- and 3,4- variants being most potent.
Table 6:Caffeic acid derivative effects on influenza1.
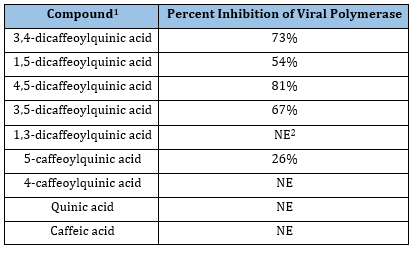
1 Lin et al. (36);
2 No effect.
Figure 7:Caffeic acid.
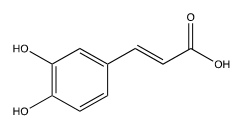
Figure 8:3,4-O-dicaffeoyl-1,5-γ-quinide, R=caffeic acid.
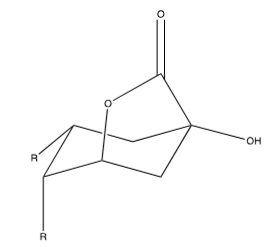
Figure 9:Quinic acid.
In cell culture with RAW264.7 cells, Ye et al. [37] found that cells pretreated with chlorogenic acid before LPS addition had lower levels of IL-6, TNF-, Macrophage Inflammatory Protein-2 (MIP-2) and IL-1. They further found that LPS injury to a mouse’s kidney could be attenuated by the addition of chlorogenic acid. They also discovered that chlorogenic acid inhibits TNF-, IL-1, TLR-4 and IL-6 production in a dose dependent manner. This inhibition was done through the inhibition of IB, and NF-B p65 activity. The research done by Kim et al.34 supports the research done by Ye et al. [37]. Kim et al. [34] pretreated RAW264.7 cells with chlorogenic acid prior to exposing them to LPS to test the suppression of cytokines and inflammatory factors by chlorogenic acid. They found that pretreatment with chlorogenic acid suppressed iNOS, Nitric Oxide (NO), IL-6, TNF-, MIP-2, IL-, and JAK2/STAT3. Therefore, chlorogenic acid is both effective against influenza virus, and blocks the cytokine pathways involved in inflammation.
Eight nutraceuticals
Black Currant leaves, and black currant berries have both been shown to have antiviral effects. Ehrhardt et al. [38] found that LADANIA067, an extract preparation from black currant berry leaves, was effective against oseltamivir-resistant strains in influenza (Table 7) in A549 cells, a human alveolar type II epithelial cell line. In addition, the virus didn’t develop resistance to LADANIA067 like it did with amantadine (an M2 channel blocker). This viral inhibition was also found in mouse models with aerosolized LADANIA067. Hemagglutination by IAV in vitro was not inhibited by the compound, nor is sialic-acid based lectin-induced hemagglutination, indicating that LADANIA067 neither occludes the sialic-acid binding sites on the virus, nor occludes sialic-acid on the respiratory cell. LADANIA067 inhibited EGFR phosphorylation when virus was present, indicating that its mechanism involves inhibiting receptor-mediated endocytosis and viral uptake into the cell. The compound neither activated NF-κB nor inhibited receptor-mediated NF-κB activation. A549 cells did not take up nor metabolize LADANIA067. It was best administered in aerosolized form. Teaupa et al. [39] found that an aqueous extract of black currant berries inhibited A/CA/07/09 (H1N1) with a half maximal response (EC50) of 0.016±0.002mosmol/kg (±SEM) in MDCK cells. It is interesting to note that Ehrhardt et al. [38] pretreated their cells with the extract, whereas Teaupa et al. [39] pretreated their cells with virus first; and in both cases the virus was inhibited. However, not too many parallels can be drawn between the two studies without further research since Teaupa et al. [39] used the berries, and Ehrhardt et al. [38] used the leaves (Table 7).
Table 7:Black Currant effects on influenza.

1 Ehrhardt et al. (38)”.
2 Osteltamivir resistant pandemic 2009 strain;
3 Percent reduction in viral titer in A549 cell culture medium in presence of compound at 50μg/ml 8 hours post infection;
4 Teaupa et al. [39];
5 EC50±SEM in MDCK cell 48-hour CPE assay.
Black currant has also been found to have anti-inflammatory properties too, despite Ehrhardt et al. [38] not finding any NF-κB inhibition with LADANIA067. Desjardins et al. [40] found that an extract with black currant was effective in alleviating toxicity caused by nicotine in oral epithelial cells. The effective concentrations they found ranged from 25-50μg/mL when the extract was used prior to nicotine. They also tested Cyanidin-3-O-glucoside, a major anthocyanin in black currant (Figure 10), for its effect against inflammation, and found that it protected the cells too. It was also found that black currant extract decreased the secretion of IL-6 by 37% in macrophages preincubated with the extract and then exposed to nicotine. Cyanidin-3-O-glucoside decreased the production of IL-6 by 22% or 46% when 5μg/mL or 25μg/mL of extract was preincubated with the macrophages prior to exposure to nicotine, respectively. Therefore, Black Currant can produce anti-inflammatory effects due to Cyanidin-3-O-glucoside, and antiinfluenza properties when prepared as the extract LADANIA067.
Table 8:Black Currant effects on influenza.

1 Desjardins et al. [40]
Figure 10:Cyanidin-3-O-glucoside.
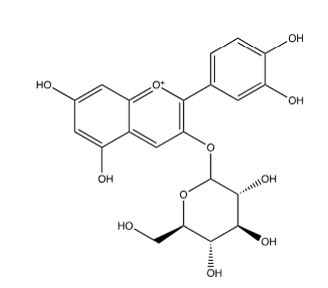
Jamaican Sorrel (Hibiscus sabdariffa), otherwise known as Roselle, has been shown to be effective against different strains of influenza. Baatartsogt et al. [41] found that an aqueous extract of Hibiscus (2 grams boiled in 100mL water for one hour, then centrifuged in a concentrator filter device) was effective against several types of influenza when the virus was mixed with the extract for either 10 seconds or 10 minutes then incubated with MDCK cells for 5 days (Table 9). They hypothesized that this block was from HA inhibition. Using MDCK cells, Teaupa et al. [39] also found that an aqueous extract of Hibiscus was effective against A/CA/07/09 (H1N1) with an EC50 of 0.0313±0.0014mmol/kg (±SEM) (Table 10). Da-Costa-Rocha et al. [42], found that Hibiscus has quercetin (Figure 3), luteolin (Figure 1), and chlorogenic acid (Figure 7), and Ramirez et al. [43] found that Hibiscus has quercetin and chlorogenic acid. Since luteolin, quercetin, and chlorogenic acid can independently inhibit influenza as discussed earlier, the combination of all three of these compounds is most likely the cause of the Hibiscus inhibition of influenza observed by Baatartsogt et al. [41] and Teaupa et al. [39].
Table 9:The Jamaican sorrel effect on influenza1.
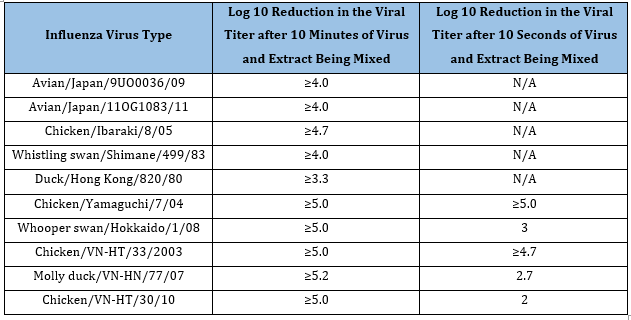
1 Baatartsgt et al. (41)
Table 10:The Jamaican sorrel effect on influenza.
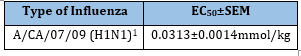
1 Teaupa et al. [39]
Jamaican sorrel also has anti-inflammatory properties, most likely due to it containing luteolin, quercetin, and chlorogenic acid as discussed. Chou et al. [44] observed that Hibiscus effectively attenuated urinary tract infections for those in long-term care facilities. To further determine the Hibiscus effect on inflammation, they injected mice with LPS and then gave them a hibiscus drink. After seven days they were sacrificed. ELISA tests were done on the serum of the mice to determine cytokine levels. A cell culture assay with a strain of immortalized hepatic cells (HepG2 cells) was also performed with LPS and Hibiscus to determine the cytokine levels. It was discovered that Hibiscus inhibits NF-κB in vitro, and IL-1β in vivo. It also suppressed IL-6, IL-22, Chemokine (C-X-C motif) ligand 2 (CXCL2), Chemokine (C-C motif) ligand 12 (CCL12), COX2 and iNOS genes. One of the key molecules involved is delphinidin- 3-sambubioside (Figure 11) which acts on NF-κB and MAPK to suppress iNOS, NO, IL-6, MCP-1 and TNF-α.
Figure 11:Delphinidin-3-Sambubioside.
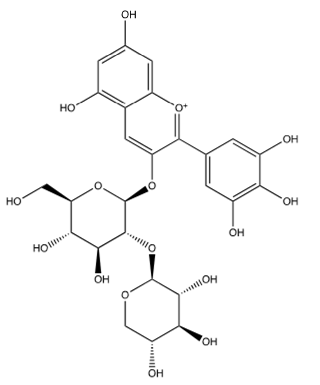
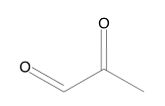
Honey: Different kinds of honey, especially Manuka honey from New Zealand have been shown to have anti-influenza properties. Watanabe et al. [45] tested manuka honey, soba honey, kanro honey, acacia honey, and renge honey as possible anti-viral agents against A/WSN/33 (H1N1) influenza in MDCK cells. Of these, manuka honey performed the best (Table 11). Soba honey, kanro honey, acacia honey and renge honey followed manuka honey for effectiveness. Charyasriwong et al. [46] found that methylglyoxal (Figure 12) was an active ingredient in manuka honey with EC50 values of 180-420 μM against H1N1, H3N2, H5N2 and even oseltamivir-resistant H1N1. They even found that manuka honey had a synergistic effect when combined with NA inhibitors like oseltamivir. Teaupa et al. [39] tested a 1:1 combination of Manuka honey and bee pollen (which contains several NA inhibitors, see bee pollen section below) and found that this combination had an EC50 of 0.0313±0.0018mmol/kg (±SEM) which was more effective than just manuka honey or bee pollen individually. Charyasriwong et al. [47], found that the mechanism of methylglyoxal may not be through inhibition of HA or NA, at least not for influenza B, but may be involved in inhibiting the NF-κB pathway due to its suppression of TNF-α. In short, the exact mechanism of methylglyoxal and manuka honey’s effect on influenza still needs to be elucidated, however they are effective against influenza, and act synergistically with NA inhibitors.
Table 11:The Jamaican sorrel effect on influenza.
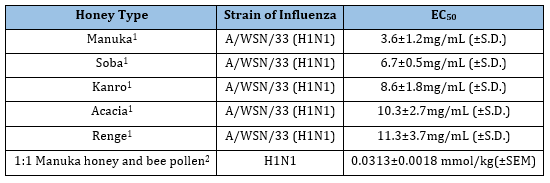
1 Watanabe et al. [45];
2 Teaupa et al. [39].
Figure 12:Methyglyoxal.
Gelam and Tualang honey have been shown to be effective against inflammation and to inhibit similar pathways influenza induces. Hussein et al. [48] studied the effects Gelam honey had on rat paw inflammation stimulated by carrageenan. They found that pretreatment of Gelam honey inhibited NF-B, IB, COX-2, and TNF- in the rat paws. Ahmad et al. [49] found that Tualang honey protected murine epidermal keratinocyte cells from UVB exposure by inhibiting NF-B and the degradation of IB. It also inhibited IL-1, IL-6, and TNF- release from PAM212 keratinocytes exposed to UVB, and decreased expression of COX-2 and Prostaglandin E2. Ahmad et al. [49] & Kogilavanee et al. [50] reviewed the literature on Taulang honey in regard to inflammation, and found several studies discussing honey’s anti-inflammatory effects. In conclusion, Tualang and Gelam honey have been shown to inhibit influenza-induced cytokines, so they could each have an effect on the cytokine storm.
The flavone kaempferol (Figure 13) is a variant of quercetin (Figure 3) in which the catechol is converted to phenol. Bee pollen is rich in kaempferol glycosides [22]. The common glycosides are shown in Figure 14-17. The “X” on each glycoside represents a hydroxyl that is eliminated in forming the ether bond to kaempferol at its 3-hydroxy group (designated as OR). As discussed earlier, Lee et al. [22] tested several different compounds for their effectiveness against influenza in general, and specifically NA. Although not as effective as luteolin, they found that four derivatives of Kaempferol were effective towards inhibiting influenza though NA inhibition (Table 12).
Figure 13:Kaempferol.
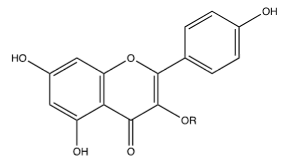
Figure 14:Kaempferol-3-Sophoroside.
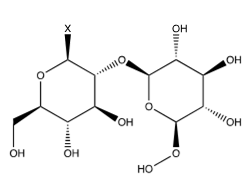
Figure 15:Kaempferol-3-Neohesperidoside.

Figure 16:Kaempferol-3-Sambubioside.
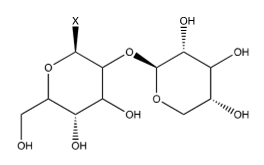
Figure 17:Kaempferol-3-Oβ-D-glucoside.
Table 12:The Jamaican sorrel effect on influenza.
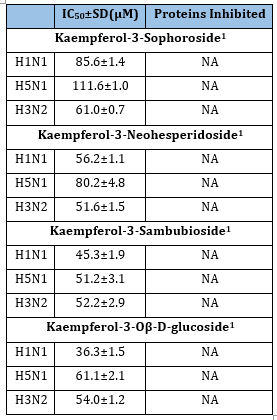
1 Lee et al. (22)
Bee pollen also has anti-inflammatory properties, inhibiting inflammatory pathways similar to those activated by influenza. Huang et al. [51] tested Schisandra chinensis bee pollen on the liver and kidneys of Sprague Dawley rats after the rats had bee pollen and cisplatin (an inflammatory agent) injected into their intraperitoneal region. They found that NF-B, IL-1, and IL-6 were decreased in the rats injected with bee pollen compared to the controls. Devi et al. [52] reviewed literature on kaempferol, observing that kaempferol reportedly inhibits COX-1, COX-2, iNOS, MAPK, Extracellular Signal-Regulated Kinases (ERK), STAT3, NF-B, TNF-, and other inflammatory pathways. Thus, kaempferol compounds augment the anti-viral and anti-inflammatory effects of luteolin and quercetin in bee pollen discussed above.
Bee propolis: Takemura et al. [53] found that Brazilian Green Propolis contains chlorogenic acid (Figure 6), caffeic acid (Figure 7), and several other compounds, including 3,4,5-tricaffeoylquinic acid, artepillin C, baccharin, p-coumaric acid, isosakuranetin, drupanin, ferulic acid. They also studied the effect of bee propolis on A/WSN/33 (H1N1) intranasally infected BALB/c mice (Table 13). They found that 3,4-dicaffeoylquinic acid (Figure 8 & 10); (Table 6) was one of the major constituents of bee propolis that gave it an antiviral effect. However, they also mentioned that other yet-to-be quantified components of bee propolis probably had an effect against influenza as well. The mechanism of this inhibition involved increased Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL), which induced the apoptosis of influenza virus infected cells; they also found that the propolis had an unknown cytoprotective mechanism. Búfalo et al. [54] found that propolis contains caffeic acid (Figure 7) and apigenin (Figure 2) which have been shown to be effective against influenza; it also contains caffeoylquinic acid, derivatives of which have been shown to inhibit influenza (Table 6); [23].
Table 13:The Bee Propolis effect on influenza.
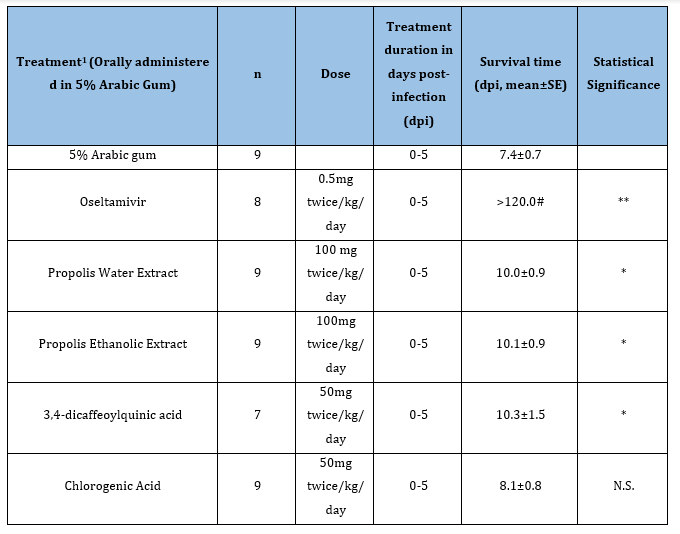
#Standard error not determined;
*P<0.05; **P<0.01; N.S. not significant.
1 Takemura et al. [53].
Bee propolis is also capable of inhibiting several cytokines associated with the cytokine storm induced by influenza. Song et al. [55] found that caffeic acid phenethyl ester (CAPE, like chlorogenic acid (Figure 6), but with phenethyl alcohol replacing quinic acid), a component of bee propolis, was effective against blocking TNF-, IL-8, and IB- in human middle ear epithelial cells when they were exposed to LPS. Búfalo et al. [54] also found that caffeic acid and propolis were effective in suppressing p38 MAPK, C-Jun N-Terminal kinase ½ (JNK1/2), NO, and NF-B in Raw 264.7 cells when they were exposed to LPS. Therefore, bee propolis is effective against influenza virus itself, and affects some of the inflammatory pathways involved in the cytokine storm.
Echinaforce: Rauš et al. [15] performed a randomized, double blind, multicenter, controlled clinical trial on 473 patients to compare Echinaforce, an Echinacea purpurea extract, against oseltamivir. Both the oseltamivir and Echinaforce groups showed a similar fever reduction after the start of treatment over a period of two days. The Echinaforce group also showed an almost statistically significant decrease in complications (P=0.076). The Echinaforce group had five times less adverse effects than the oseltamivir group; these effects were usually gastrointestinal issues like nausea and vomiting. Schapowal et al. [56] indicated that Echinaforce affects HA and NA, and that influenza doesn’t get resistant to Echinaforce like it does to oseltamivir [57]. In fact, oseltamivir resistant virus was still susceptible to Echinaforce. Sharma et al. [58] found that Echinaforce inhibited H3N2 infections of MDCK cells in the CPE assay (Table 14). Pleschka et al. [53] used MDCK cells to test Echinaforce inhibition of H3N2 (Table 15), as well as H1N1, H5N1, and H7N7. They observed that the extract could inactivate 99% or more of the virus at concentrations ranging from 0.16 to 53.3μg/ml. Pleschka et al. [57] also determined that Echinaforce inhibited HA (Table 16) using a hemagglutination assay.
Table 14:The Echinaforce effect on influenza H3N2.
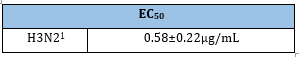
1 Takemura et al. [53].
Table 15:The Echinaforce effect on influenza H3N2 virus titer.
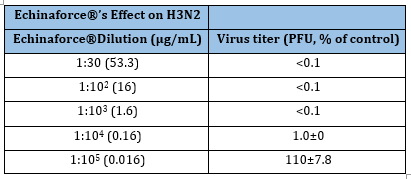
1 Sharma et al. [58]
Table 16:The Echinaforce effects on influenza HA protein
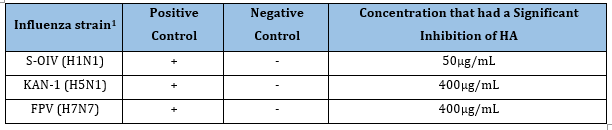
1 Pleschka et al. [57].
Echinaforce also has anti-inflammatory effects. Sharma et al. [58] found that Echinaforce blocked release of CXCL1 (GRO-α), CXCL8 (IL-8), CCL2 (MCP-1), CCL5 (RANTES), IL-1α, IL-5, IL-6, TNF-α, IL-6, and IL-8 in BEAS-2 (human bronchial epithelial) cells exposed to influenza. Vimalanathan et al. [59] used BEAS-2 cells to determine how well Echinaforce blocks virus-upregulated bacterial adhesion receptors on the cell surface for H. influenzae and S. aureus to prevent these bacteria from causing a superinfection (which is usually pneumonia). It was found that Echinaforce does indeed inhibit expression of these receptors, so it could inhibit these bacteria from getting to the bronchial cells and infecting them too. They also found that Echinaforce reduced NF-B, IL-6, and IL-8 in cells exposed to LPS. Sharma et al. [58] found that Echinaforce contains chlorogenic acid (Figure 6), caffeic acid (Figure 7), and several caffeic-acid containing compounds, namely echinacoside (Figure 18), cynarin (Figure 19), caftaric acid (Figure 20), and cichoric acid (Figure 21), which, given their caffeic acid components, may be responsible for Echinaforce’s effects on influenza and inflammation.
Figure 18:Echinacoside.

Figure 19:Cynarin, R=caffeic acid.
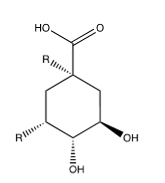
Figure 20:Caftaric acid, R= caffeic acid.
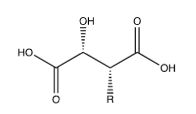
Figure 21:Cichoric acid, R= caffeic acid.

Goldenseal: It has anti-influenza properties due to berberine (Figure 22). Cecil et al. [60] tested this isoquinoline alkaloid, a component of goldenseal, barberry (Berberis vulgaris), coptis (Coptis chinensis), and Oregon grape (Mahonia aquifolium), on H1N1 in RAW264.7 macrophage-like cells, A549 human lung epithelial-derived cells, murine bone marrow derived macrophages, and MDCK cells. Berberine was effective at inhibiting the virus production in all the cell lines except for the MDCK cells. In RAW 264.7 cells, berberine had EC50 values of 0.01 and 0.44μM for the two H1N1 virus strains tested, much lower than amantadine which had an EC50 of 27μM (Table 17). They found that the mechanism behind this inhibition is most likely related to post-translational effects on transport or maturation of viral proteins inside the cell.
Table 17:The Berberine effect on influenza.
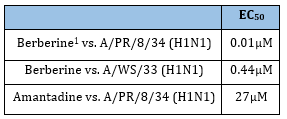
1 Cecil et al. [60].
Figure 22:Berberine.
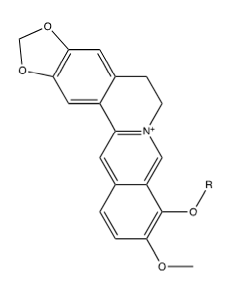
Enkhtaivan et al. [61] tested berberine-piperazine derivatives for their effects on A/PR/8/34 (H1N1), A/Vic/3/75 (H3N2), B/Lee/40, and B/Maryland/1/59 strains of influenza in MDCK cells compared to oseltamivir. Table 18 shows results for the five derivatives berberine with adducts attached as ethers to its “OR” via their terminal pentyl carbons, shown in Figure 23-27 found to be most effective against A/PR/8/34 (H1N1). They also tested the binding affinity of the different compounds to NA. Cecil et al. [60] also found that Berberine and goldenseal extracts inhibit TNF-, and PGE2. Li et al. [59] found that berberine decreased IL-1, IL-4, IL-5, IL-6, IL-13, IL-17 through inhibiting NF-B p65, p-NF-B p65 and p-IB in a dose dependent manner. These tests were done with male Wister rats that had ovalbumin injected into their intraperitoneal cavity to induce asthma [62]. Therefore, Goldenseal in general, and specifically the root, is effective against influenza virus infection and the resulting cytokine storm mostly due to it containing berberine and derivatives thereof.
Table 18:The Berberine-Piperizine1 derivative effects2 on influenza.
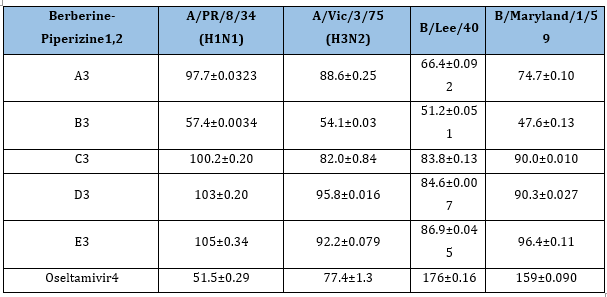
1 Enkhtaivan et al. [61];
2 EC50±experimental uncertainty (μM).
3 Berberine adducts are the piperizine variants shown in Figure 24-28.
4 Oseltamivir phosphate ester, i.e. Tamiflu, which is weakly active in cell culture compared to the active agent, oseltamivir phosphate carboxylate.
Figure 23:Berberine adduct A.
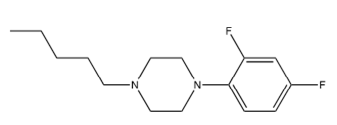
Figure 24:Berberine adduct B.
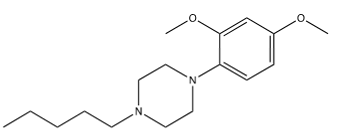
Figure 25:Berberine adduct C.
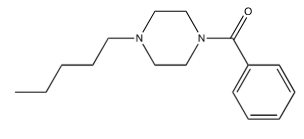
Figure 26:Berberine adduct D.
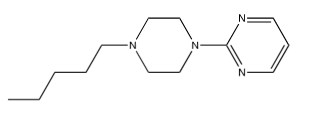
Figure 27:Berberine adduct E.

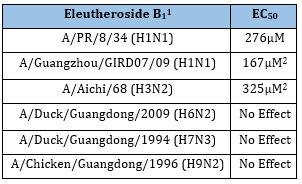
Siberian Ginseng: Otherwise known as Acanthopanax senticosus, or Eleutherococcus senticosus, has anti-influenza properties through a number of flavonoids it contains. Liu et al. [63] ran an ultra-performance liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry to determine the various components of Siberian Ginseng [64]. They found that it contained syringin (also known as eleutheroside B, Figure 28), chlorogenic acid (Figure 6), caffeic acid (Figure 7), eleutheroside E (Figure 29), and isofraxidin (Figure 30). Yang et al. [34] found that it contained eleutheroside B1 (Figure 31). Wang et al. [65] used MDCK cells to find that eleutheroside B1 was effective against A/PR/8/34 (H1N1), A/Guangzhou/GIRD07/09 (H1N1), and A/Aichi/68 (H3N2), but it had no effect on avian influenza viruses like A/Duck/Guangdong/2009 (H6N2), A/Duck/Guangdong/1994 (H7N3), or A/Chicken/Guangdong/1996 (H9N2) (Table 19). Therefore, it is reasonable to conclude that eleutheroside B1 inhibits influenza by a hemagglutinin specific mechanism. For Siberian Ginseng extracts, this could combine with anti-viral effects from chlorogenic acid, eleuthroside B and some of its other components.
Table 19:The Eleutheroside B1 effects on influenza1;
1Wang et al. [65]
2 These units, μM, are based on corrections to the published units, which are clearly erroneous, i.e. high by a factor of 1000.
Figure 28:Syringin.

Figure 29:Eleutheroside E.

Figure 30:sofraxidin.

Figure 31:Eleutheroside B1..

Siberian Ginseng also confers some anti-inflammation effects. Wang et al. [65] found that eleutheroside B1 suppressed influenza-induced expression of CXCL-8, CCL-2 and IL-6 in A549 cells. Han et al. [66] injected piglets with LPS or saline in the intraperitoneal cavity. They then gave Siberian Ginseng polysaccharides (800mg/kg dietary supplement) to half of the piglets and not to the control group. They determined that Siberian Ginseng decreased expression of IL-1, IL-6, and TNF-. Therefore, Siberian Ginseng may have a similar effect on inflammation due to eleutheroside B1, and chlorogenic acid; some of the other components may play a role as well.
Conclusion
In conclusion, influenza is a major disease that usually just causes minor symptoms, but it can prove fatal. The systemic symptoms are due to the release of cytokines by the body. Traditionally oseltamivir is used to treat influenza infections. In particular, Echinaforce® performed admirably in a clinical trial against oseltamivir. But there are other compounds that may also be effective against influenza and may also aid in abating the cytokine storm. Some of these compounds include luteolin, apigenin, quercetin, and chlorogenic acid. These compounds and others can be found in various nutraceuticals like the eight described in this paper. It should be noted though that there is some thought that these compounds, and other catechol and phenolic compounds may constitute Pan-Assay Interference Compounds (PAINS) which have a variety of binding sites, but not specific activity against one protein [67]. Therefore, these compounds may be useful for developing a combo-therapy since they work in vitro, however they are not as beneficial for guiding medicinal chemistry (which is focused on improving the potencies and patentability of different compounds) since these compounds have non-specific binding sites. Perhaps through combining these compounds with others that have shown effective against influenza, more effective drug combinations could be made to treat this potentially deadly disease.
References
- Center for Disease Control and Prevention (2019) Disease burden of influenza.
- Center for Disease Control and Prevention (2019) Key facts about influenza (Flu).
- Eierhoff T, Hrincius ER, Rescher U, Ludwig S, Ehrhardt C (2010) The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLOS Pathogens.
- Vrijens P, Noppen S, Boogaerts T, Vanstreels E, Ronca R, et al. (2019) Influenza virus entry via the GM3 ganglioside-mediated platelet-derived growth factor receptor beta signaling pathway. The Journal of General Virology 100(4): 583-601.
- Rudnicka A, Yamauchi Y (2016) Ubiquitin in influenza virus entry and innate immunity. Viruses 8(10): 293.
- Garcia J, Lai JC, Haselhorst T, Choy KT, Yen H, et al. (2013) Investigation of the binding and cleavage characteristics of N1 neuraminidases from avian, seasonal and pandemic influenza viruses using saturation transfer difference nuclear magnetic resonance. Influenza and Other Respiratory Viruses 8(2): 235-242.
- Arancibia SA, Beltrán CJ, Aguirre IM, Silva P, Peralta AL, et al. (2007) Toll-like receptors are key participants in innate immune responses. Biological Research 40(2): 97-112.
- He L, Fan F, Hou X, Wu H, Wang J, et al. (2017) 4(3H)-Quinazolone regulates innate immune signaling upon respiratory syncytial virus infection by moderately inhibiting the RIG-1 pathway in RAW264.7 cell. International Immunopharmacology 52: 245-252.
- Lee DW, Faubel S, Edelstein CL (2015) A pan caspase inhibitor decreases caspase-1, IL-1 α and IL-1 β, and protects against necrosis of cisplatin-treated freshly isolated proximal tubules. Renal Failure 37(1): 144-150.
- Pinto R, Herold S, Cakarova L, Hoegner K, Lohmeyer J, et al. (2011) Inhibition of influenza virus-induced NF-κB and ERK activation can simultaneously reduce both, virus titres and cytokine expression in vitro and in vivo. Antiviral Research 92(1): 45-56.
- Ding Y, Chen L, Wu W, Yang J, Yang Z, et al. (2017) Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microbes and Infection 19(12): 605-615.
- Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB (2014) Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proceedings of the National Academy of Sciences 111(10): 3799-3804.
- Gao S, Malsburg A, Dick A, Faelber K, Schröder G, et al. (2011) Structure of myxovirus resistance protein A reveals intra-and intermolecular domain interactions required for the antiviral function. Immunity 35(4): 514-525.
- Handfield C, Kwock J, MacLeod A (2018) Innate antiviral immunity in the skin. Trends in Immunology 39(4): 328-340.
- Rauš K, Pleschka S, Klein P, Schoop R, Fisher P (2015) Effect of an echinacea-based hot drink versus oseltamivir in influenza treatment: A randomized, double-blind, double-dummy, multicenter, noninferiority clinical trial. Current Therapeutic Research 77: 66-72.
- Wang XY, Jia W, Zhao AH, Wang XR (2006) Anti-influenza agents from plants and traditional Chinese medicine. Phytotherapy Research 20(5): 335-341.
- Hudson JB (2009) The use of herbal extracts in the control of influenza. Journal of Medicinal Plants Research 13(3): 1189-1195. ISSN: 1996-0875
- Bahramsoltani R, Sodagari HR, Farzaei MH, Abdolghaffari AH, Gooshe M, et al. (2016) The preventive and therapeutic potential of natural polyphenols on influenza. Expert Review of Anti-Infective Therapy 14(1): 57-80.
- Ide K, Kawasaki Y, Kawakami K, Yamada H (2016) Anti-influenza virus effects of catechins: A molecular and clinical review. Current Medicinal Chemistry 23(42): 4773-4783.
- Zakaryan H, Arabyan E, Oo A, Zandi K (2017) Flavonoids: promising natural compounds against viral infections. Archives of Virology 162(9): 2539-2551.
- Michalowska AG, Sidor A, Kulczynski B (2017) Berries as a potential anti-influenza factor - A review. Journal of Functional Foods 37: 116-137.
- Lee I, Hwang B, Kim D, Kim J, Woo E, et al. (2016) Characterization of neuraminidase inhibitors in Korean Papaver Rhoeas bee pollen contributing to anti-influenza activities in vitro. Planta Medica 82(6): 524-529.
- Liu A, Liu B, Qin H, Lee S, Wang Y et al. (2008) Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa. Planta Medica 74(08): 847-851.
- Sithisarn P, Michaelis M, Schubert ZM, Cinatl J (2013) Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Research 97(1): 41-48.
- Jia Z, Nallasamy P, Liu D, Shah H, Li JZ, et al. (2015) Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. The Journal of Nutritional Biochemistry 26(3): 293-302.
- Pratheesh KP, Son YO, Divya SP, Roy RV, Hitron JA, et al. (2014) Luteolin inhibits Cr (VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicology and Applied Pharmacology 281(2): 230-241.
- Basios N, Lampropoulos P, Papalois A, Lambropoulou M, Pitiakoudis MK (2016) Apigenin attenuates inflammation in experimentally induced acute pancreatitis-associated lung injury. Journal of Investigative Surgery 29(3): 121-127.
- Wrobel MP, Borkowska P, Samojedny MP, Kowalczyk M, Danilow AF (2017) Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomedicine & Pharmacotherapy 93: 1205-1212.
- Wu W, Li R, Li X, He J, Jiang S, et al. (2015) Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 8(1): 6.
- Choi HJ, Song JH, Park KS, Kwon DH (2009) Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. European Journal of Pharmaceutical Sciences 37(3-4): 329-333.
- Shin JS, Ku KB, Jang Y, Yoon YS, Shin D, et al. (2017) Comparison of anti-influenza virus activity and pharmacokinetics of oseltamivir free base and oseltamivir phosphate. Journal of Microbiology (Seoul, Korea) 55(12): 979-983.
- Takashima K, Matsushima M, Hashimoto K, Nose H, Sato M, et al. (2014) Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury. Respiratory Research 15(1): 150.
- Maturu P, Liang WY, Androutsopoulos VP, Jiang W, Wang L, et al. (2018) Quercetin attenuates the hyperoxic lung injury in neonatal mice: Implications for bronchopulmonary dysplasia (BPD). Food and Chemical Toxicology 114: 23-33.
- Kim S, Park S, Park Y, Myung D, Rew J et al. (2017) Chlorogenic acid suppresses lipopolysaccharide-induced nitric oxide and interleukin-1β expression by inhibiting JAK2/STAT3 activation in RAW264.7 cells. Molecular Medicine Reports 16(6): 9224-9232.
- Sinisi V, Stevaert A, Berti F, Forzato C, Benedetti F et al. (2016) Chlorogenic compounds from coffee beans exert activity against respiratory viruses. Planta Medica 83(7): 615-623.
- Lin L, Shenghai C, Junfeng X, Qian L, Huanhuan L et al. (2012) Screen anti-influenza lead compounds that target the PA(C) subunit of H5N1 viral RNA polymerase. Plos ONE 7(8): 1-7.
- Ye H, Jin J, Jin L, Chen Y, Zhou Z et al. (2017) Chlorogenic acid attenuates lipopolysaccharide-induced acute kidney injury by inhibiting TLR4/NF-κB signal pathway. Inflammation 40(2): 523-529.
- Ehrhardt C, Dudek SE, Holzberg M, Urban S, Hrincius ER, et al. (2013) A plant extract of Ribes nigrum folium possesses anti-influenza virus activity in vitro and in vivo by preventing virus entry to host cells. PLoS ONE 8(5): e63657.
- Teaupa S, Williamson M, Humpherys B, Busath D (2018) Nutraceuticals: An alternative treatment for influenza virus. Scholars Archive.
- Desjardins J, Tanabe S, Bergeron C, Gafner S, Grenier D (2012) Anthocyanin-rich black currant extract and cyanidin-3-o-glucoside have cytoprotective and anti-inflammatory properties. Journal of Medicinal Food 15(12): 1045-1050.
- Baatartsogt T, Bui VN, Trinh DQ, Yamaguchi E, Gronsang D et al. (2016) High antiviral effects of hibiscus tea extract on the H5 subtypes of low and highly pathogenic avian influenza viruses. Journal of Veterinary Medical Science 78(9): 1405-1411.
- Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L.-a phytochemical and pharmacological review. Food Chemistry 165: 424-443.
- Ramirez RMM, Plaza ML, Azeredo A, Balaban MO, Marshall MR (2011) Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. Journal of Food Science 76(3): 428-435.
- Chou S, Lo H, Li C, Cheng L, Chou P, et al. (2016) Exploring the effect and mechanism of Hibiscus sabdariffa on urinary tract infection and experimental renal inflammation. Journal of Ethnopharmacology 194: 617-625.
- Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, Kobayashi N (2014) Anti-influenza viral effects of honey in vitro: potent high activity of manuka honey. Archives of Medical Research 45(5): 359-365.
- Charyasriwong S, Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, et al. (2015) In vitro evaluation of synergistic inhibitory effects of neuraminidase inhibitors and methylglyoxal against influenza virus infection. Archives of Medical Research 46(1): 8-16.
- Charyasriwong S, Haruyama T, Kobayashi N (2016) In vitro evaluation of the antiviral activity of methylglyoxal against influenza B virus infection. Drug Discoveries and Therapeutics 10(4): 201-210.
- Hussein SZ, Yusoff KM, Makpol S, Yusof YA (2013) Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-κB pathway. PLoS ONE 8(8): 1-12.
- Ahmad I, Jimenez H, Yaacob NS, Yusuf N (2012) Tualang honey protects keratinocytes from ultraviolet radiation-induced inflammation and DNA damage. Photochemistry and Photobiology 88(5): 1198-1204.
- Kogilavanee D, Yong Y (2016) Anti-inflammatory and wound healing properties of Malaysia Tualang Honey. Current Science 110(1): 47-51.
- Huang H, Shen Z, Geng Q, Wu Z, Shi P, et al. (2017) Protective effect of Schisandra chinensis bee pollen extract on liver and kidney injury induced by cisplatin in rats. Biomedicine and Pharmacotherapy 95: 1765-1776.
- Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J (2015) Kaempferol and inflammation: From chemistry to medicine. Pharmacological Research 99: 1-10.
- Takemura T, Urushisaki T, Fukuoka M, Muto JH, Hata T, et al. (2012) 3,4-Dicaffeoylquinic acid, a major constituent of Brazilian propolis, increases TRAIL expression and extends the lifetimes of mice infected with the influenza A virus. Evidence-Based Complementary and Alternative Medicine 1-7.
- Búfalo MC, Ferreira I, Costa G, Francisco V, Liberal J, et al. (2013) Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. Journal of Ethnopharmacology 149(1): 84-92.
- Song J, Cho JG, Hwang S, Cho CG, Park S, et al. (2008) Inhibitory effect of caffeic acid phenethyl ester (CAPE) on LPS-induced inflammation of human middle ear epithelial cells. Acta Oto-Laryngologica 128(12): 1303-1307.
- Schapowal A (2012) Efficacy and safety of echinaforce® in respiratory tract infections. Wiener Medizinische Wochenschrift 163(3-4): 102-105.
- Pleschka S, Schoop R, Hudson JB (2011) Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and Swine-origin H1N1 (S-OIV). Antiviral Research 90(2): 197-205.
- Sharma M, Anderson SA, Schoop R, Hudson JB (2009) Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antiviral Research 83(2): 165-170.
- Vimalanathan S, Schoop R, Suter A, Hudson J (2017) Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Research 233: 51-59.
- Cecil CE, Davis JM, Cech NB, Laster SM (2011) Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis). International Immunopharmacology 11(11): 1706-1714.
- Enkhtaivan G, Kim DH, Park GS, Pandurangan M, Nicholas DA, et al. (2018) Berberine-piperazine conjugates as potent influenza neuraminidase blocker. International Journal of Biological Macromolecules 119: 1204-1210.
- Li Z, Zheng J, Zhang N, Li C (2016) Berberine improves airway inflammation and inhibits NF-κB signaling pathway in an ovalbumin-induced rat model of asthma. Journal of Asthma 53(10): 999-1005.
- Liu S, An J, Wang R, Li Q (2012) Simultaneous quantification of five bioactive components of Acanthopanax senticosus and its extract by ultra-performance liquid chromatography with electrospray ionization time-of-flight mass spectrometry. Molecules 17(7): 7903-7913.
- Yang L, Ge H, Wang W, Zu Y, Yang F, et al. (2013) Development of sample preparation method for Eleutheroside B and E analysis in Acanthopanax senticosus by ionic liquids-ultrasound based extraction and high-performance liquid chromatography detection. Food Chemistry 141(3): 2426-2433.
- Wang Y, Yan W, Chen Q, Huang W, Yang Z, et al. (2017) Inhibition viral RNP and anti-inflammatory activity of coumarins against influenza virus. Biomedicine and Pharmacotherapy 87: 583-588.
- Han J, Bian L, Liu X, Zhang F, Zhang Y, et al. (2014) Effects of Acanthopanax senticosus polysaccharide supplementation on growth performance, immunity, blood parameters and expression of pro-inflammatory cytokines genes in challenged weaned piglets. Asian-Australasian Journal of Animal Sciences 27(7): 1035-1043.
- Baell JB (2016) Feeling nature's PAINS: natural products, natural product drugs and pan assay interference compounds (PAINS). The Journal of Natural Products 79(3): 616-628.
© 2019 Brayden Humpherys. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






