- Submissions

Full Text
Advances in Complementary & Alternative medicine
Alternative Therapies for Liver Diseases
Yi-Seong Kwak*, Jong-Soo Kyung and Jae-Joon Wee
The Korean Ginseng Research Institute, South Korea
*Corresponding author:Yi-Seong Kwak, The Korean Ginseng Research Institute, Korea Ginseng Corporation (KGC), 30 Gajeong-ro, Yuseong-gu, Daejeon 305-805, South Korea
Submission: May 08, 2019;Published: May 28, 2019

ISSN: 2637-7802 Volume4 Issue3
Abstract
Objectives: The present study investigated the anti-obesity activities of red ginseng acidic polysaccharide (RGAP) from Korean red ginseng (Panax ginseng C.A. Meyer) in high-fat diet (HFD)-induced mice.
Methods: Forty mice were divided randomly into four group (n=10, respectively): normal control (NC), high-fat-diet (HFD), HFD plus RGAP300mg/kg, HFD plus RGAP500mg/kg. The normal control was fed a normal diet and the other groups were fed an HFD. HFD mice were made obese by high-fat diet (35% fat) feeding for 6 weeks. The test mice were given red ginseng acidic polysaccharide orally at a single dose per day. Body weights of the mice were measured at weekly intervals. The effects of RGAP on obesity functions were assessed by measuring the serum lipid profiles and biomarkers of obesity. In addition, abdominal fat volumes were measured at the end of the experiment by using Micro X-ray CT.
Results: Mice in the HFD group showed an increase in body weight and food efficiency ratio, which means body weight gain per food intake. However, RGAP significantly reduced these values when compared with the normal control group. The RGAP group also showed significantly decreased epididymal fat mass. An increase in the serum levels of triglyceride and LDL/HDL ratio were observed in the HFD group, but RGAP administration reduced these serum levels. Serum levels of hepatic function markers such as AST and ALT, which were elevated by HFD-feeding, were also significantly reduced in the RGAP group. Levels of leptin, adiponectin, and lipoprotein lipase (LPL), which regulate glucose and lipid metabolism, were impaired by HFD. RGAP brought these levels back to near normal levels. In addition, it was confirmed by Micro X-ray CT that the abdominal fat masses increased by the HFD were reduced by RGAP.
Conclusion: This study showed that RGAP protected mice from obesity in the HFD-fed group. RGAP exerts anti-obesity effects in mice via the activation of lipoprotein lipase and improvement of leptin and adiponectin, which carry out critical functions in energy and lipid metabolism. These results suggest that red ginseng acidic polysaccharide might be a preventative functional food for these metabolic disorders.
Keywords: Panax ginseng C.A. Meyer; Red ginseng acidic polysaccharide; High-fat diet; Anti-obesity; Lipoprotein lipase; Abdominal fat mass; Micro X-ray CT
Abbreviations: HFD: High-Fat Diet; RGAP: Red Ginseng Acidic Polysaccharide; LDL: Low-Density Lipoprotein Cholesterol; HDL: High-Density Lipoprotein Cholesterol; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; LPL: Lipoprotein Lipase
Introduction
Korean ginseng (Panax ginseng C.A. Meyer) has been known to be a valuable food and has been traditionally used as an important herbal medicine in East Asian countries including Korea, China, and Japan for thousands of years. The ginseng root is traditionally used as an adaptogen as it is stated to have the capacity to normalize body functions and strengthen systems that are compromised by stress [1]. Adaptogens are reported to have a protective effect on health against a wide variety of environmental assaults and emotional conditions [2-5]. Fresh ginseng degrades easily at room temperature. Consequently, ginseng is processed into red ginseng through the process of steaming. The steaming and drying ginseng undergoes to become red ginseng produces changes to its chemical profile. Red ginseng and ginseng are individually regulated in Korea, Japan, and China. It has been reported that ginseng and red ginseng have similar biological effects because they come from the same part of the plant but are processed differently [1].
The main biological activities of Korean red ginseng are known to include enhancement effects such as the recovery of vital energy as well as the alleviation of fatigue, blood flow improvement, antioxidant effects, memory enhancement, and the alleviation of menopausal disorder [6-10], as well as a positive effect on antihyperlipidemic disorder [11]. The effects of ginseng on obesity have also been reported [12,13]. Panax ginseng is indeed being widely used to treat numerous diseases such as cancer, diabetes, and cardiovascular diseases [14]. Active constituents with curative features found in most ginseng species include ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids [15]. Of these, ginsenosides, a group of saponins with a triterpenoid dammarane structure, have been studied as ginseng’s primary pharmacological components with a variety of effects such as anti-diabetic, anti-cancer, anti-inflammatory, antihyperlipidemic, and anti-atherosclerosis activities [15,16].
Polysaccharide fractions from ginseng, which is its second major active component, have also been explored to understand ginseng’s biological roles in pharmacology in terms of immunostimulatory functions [15]. Numerous studies have revealed that polysaccharides from Panax ginseng have mitogenic activities, anti-tumor activities, and immunostimulating activities in cyclophosphamide-treated immunosuppressed mice [15,17- 19]. Polysaccharides are compounds consisting of numerous monosaccharides and have molecular weights ranging from tens to thousands. Polysaccharides have recently been shown to have various physiological activities. Among them, Panaxan A-U (21 kinds) [20], which has defense mechanisms, and polysaccharides with anti-complementary properties [21] have been isolated from Korean ginseng. It has also been reported that red ginseng acidic polysaccharide (RGAP) from Korean red ginseng was able to up-regulate immunostimulating and antitumor activities for the activation of natural killer cells and nitric oxide production in macrophages and in tumor-bearing models [22,23]. RGAP has been known as an immunostimulator and anti-tumor activator. Recently, it was also reported that RGAP plays a role in reducing hyperlipidemic conditions in both exogenous and endogenous short-term animal models [11]. It has been also reported that acidic polysaccharides inhibit the activity of toxohormone-L, one of the toxins that accelerates lipolysis [24] and have medicinal effects of enhancing one’s immune status.
Obesity is a serious health problem that has become prevalent in developed countries in recent years and is a risk factor for metabolic disease [25]. Some studies of Korean ginseng and red ginseng on lipid metabolism such as hyperlipidemia and hypercholesteremia have been also reported. In animal tests, saponins from ginseng block the absorption of fat and cholesterol, thereby promoting metabolism [26,27]. In a study on humans involving administration of red ginseng for 4 weeks, body fat was decreased. A study conducted among obese women in their 20s also delivered a similar result. Korean red ginseng lowers total cholesterol, triglycerides, and low-density lipoprotein in blood and expedites the breaking down of body fat and is thus effective in preventing and treating hyperlipidemia [28]. Korean red ginseng attenuated hypercholesterolemia-enhanced platelet aggregation in high-cholesterol-diet-fed rabbits [29], and Korean red ginseng extract also showed hepato-protective effects which were associated with anti-obesity effects in mice fed a high-fat-diet (HFD).
Recently, many studies have focused on evaluating the effects of Panax ginseng on obesity, hyperlipidemia, and metabolic diseases. Ginseng saponin has been shown to exert antiobesity effects in animal models fed an HFD via the inhibition of pancreatic lipase activity, leading to the reduction of intestinal absorption of dietary fat [30]. It has also been shown to regulate the hypothalamic expression of orexigenic neuropeptide Y and anorexigenic cholecystokinin in HFD groups [31]. The alteration of lipid profiles such as triglyceride and cholesterol were reversed by ginseng treatment in hyperlipidemic animals with the activation of lipoprotein lipase [32]. Several studies on the anti-obesity effects of ginseng have also been conducted at the molecular level; ginsenoside-Rh2 and ginsenoside-Rg3 effectively inhibited adipocyte differentiation via peroxisome proliferator-activated receptor (PPAR)-γ inhibition and adenosine monophosphateactivated protein kinase (AMPK) activation. The results suggested that ginsenoside-Rh2 and ginsenoside-Rg3 might contribute to the anti-obesity effect of ginseng via the regulation of PPAR-γ and AMPK signaling [29,33]. Ginsenoside-Re has shown anti-hyperlipidemic effects in mice fed an HFD with antioxidant effects as one of its action mechanisms [34].
Regarding the non-saponin component, it has been reported that non-saponin fractions containing polysaccharide are capable of inhibiting epinephrine-induced lipolysis and of stimulating insulin-mediated lipogenesis from glucose in rat adipocytes. Polysaccharides from Korean ginseng were found to inhibit toxohormone L-induced lipolysis [33]. Polysaccharides from Korean red ginseng modulated pancreatic lipase activity and caused a reduction of plasma triglyceride levels, implying the involvement of pancreatic lipase in the reduction of lipolysis [34]. In another study, Korean red ginseng polysaccharide significantly reduced triglycerides in both exogenous and endogenous short-term animal models via lipoprotein lipase activation [11].
Although several reports have indicated the role of ginseng saponin on lipid metabolism and hyperlipidemia in vivo, the effect of red ginseng polysaccharide on lipid metabolism in high obesity conditions induced by an HFD has not been elucidated yet. In this study, therefore, we aimed to explore the anti-obesity effect of red ginseng acidic polysaccharide in HFD-fed mice.
Material and Methods
Preparation of red ginseng acidic polysaccharide (RGAP)
RGAP was isolated from Korean red ginseng as described previously [35]. Briefly, Korean red ginseng powder was percolated with 5 volumes of 70% ethanol to extract ethanol-soluble materials. Remaining residues were then percolated with 5 volumes of distilled water, and the resulting water-soluble fractions were concentrated by vacuum evaporation. The concentrates underwent ultrafiltration to completely cut off small molecules less than 10kDa (Ultrafiltration kit, Millipore, Pellicone 2, Bedford, M.A, USA) and to obtain the non-ultrafiltrated fraction consisting of acidic polysaccharide (yield: about 15%). One milligram of RGAP contained less than 0.006EU of endotoxin [11].
Animals and diets
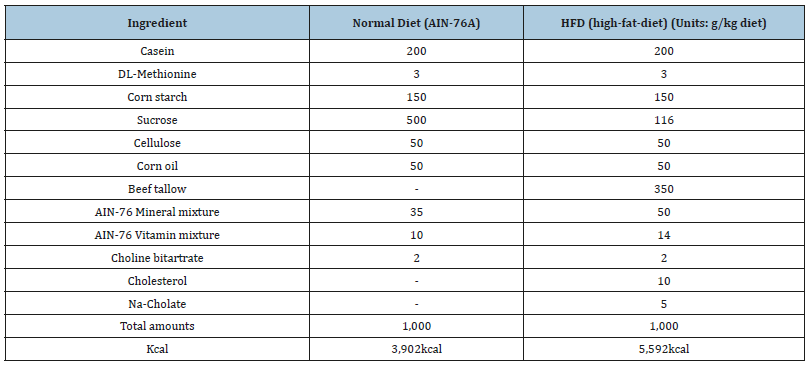
Specific pathogen-free mice (ICR, male, 5 week, 25±2g) were purchased from Charles River Laboratories, Japan and maintained in a temperature and humidity-controlled room on a 12-hour lightdark cycle. The mice were permitted to acclimate to the facility for one week and were housed at two heads per cage with ad libitum access to food and water. The forty mice were divided randomly into four groups (n=10, respectively): normal control (NC), highfat- diet (HFD), HFD plus RGAP 300mg/kg, and HFD plus RGAP 500mg/kg. The normal group was fed a normal diet (AIN-76A), and the other groups were fed an HFD. The normal diet contained 3,902 kcal, whereas the HFD contained 5,592kcal and 35% fat (Table 1). The normal diet (AIN-76A) and HFD diet were custom-formulated by Feed Lab Korea Co., Ltd (Hanamm, Gyeonggi, Korea). The mice received the experimental diets for 6 weeks, and RGAP groups (R300, R500) received a dose of 300mg/kg/day and 500mg/kg/ day, respectively.
Body weight and food intake
Body weights were measured once per week, and food intake was recorded every three days. The results of body weight and food intake are shown in Figure 1 and Table 2.
Figure 1:Effect of RGAP on body weight in HFD-fed mice. NC: Normal Control; HFD: High-Fat Diet group; R300: HFD+RGAP300mg/kg group; R500: HFD+RGAP500mg/ kg group; #significant p<0.05: HFD vs R500
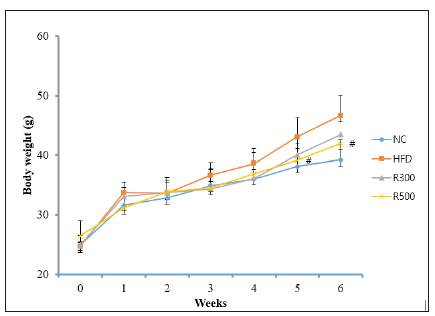
Table 1:Composition of experimental diets.
Micro X-ray CT (computed tomography) analysis
The mice were subjected to micro X-ray CT imaging (DRGEM, Harmony 130G, Korea). Tube voltage was set at 130kV, and the mice were scanned with a rotating beam (cone beam CT method). Micro X-ray CT is a non-destructive technique that allows visualization of the internal structure of mice, determined mainly by variations in density and atomic composition. This is followed by the reconstruction of two-dimensional cross-sections perpendicular to the axis of rotation [36]. The Micro X-ray CT was carried out on three mice in each group. Abdominal fat mass in mice were represented as percent of image area. The area percent was analyzed by computer area analysis between groups.
Organ weight and lipid concentrations
At the end of the feeding period, the mice were placed on overnight fasting. After collecting the blood sample, the epididymal fat, liver, and other organs were immediately excised and weighed. Blood was extracted from the heart and centrifuged for 10min at 300×g. All serum was stored at -70 °C until assayed for the biological parameters. The total cholesterol (TC), triglyceride (TG), aminotransferase (AST), alanine aminotransferase (ALT), lowdensity lipoprotein (LDL), and high-density lipoprotein (HDL) were measured using commercial kits from Wako (Japan) using a Hitachi 7020 analyzer. The serum leptin and adiponectin concentrations were determined with an ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA).
LPL activity assay
LPL activity (lipoprotein lipase) was measured from plasma using ELISA (Daichi Pure Chemical Company, Tokyo, Japan). The activity was expressed in units representing nmol of free fatty acids released per ml of plasma.
Statistical Analysis
Body weights in normal control (NC), HFD and HFD plus RGAP groups (R300, R500) were compared using one-way ANOVA followed by Duncan’s test for multiple comparisons. All results were expressed as means±SD. Values of p<0.05 were considered significant, if not otherwise stated.
Results
Body weight and food intake
Feeding mice with the HFD containing 35% beef tallow caused a marked increase in body weight as compared to feeding them a normal diet. However, the HFD plus RGAP administration significantly reduced the weight gain induced by the HFD. Six weeks of feeding on the HFD increased the body weights of the mice from 24.7±1.1g to 45.6±2.2g, whereas the body weights of the normal control group changed from 25.0±1.2g to 39.2±1.0g. Limited increases were observed in the body weights of the mice in the HFD plus RGAP groups (R300, R500), going from 25.0±1.2g to 43.4±1.1g and from 26.4±1.0 to 42.9±1.5g, respectively. Food intake was not altered by the HFD or HFD plus RGAP administration. The food efficiency ratio was increased in the HFD group compared to the normal control group (NC), but administration of RGAP reduced that value significantly (Table 2). RGAP administration inhibited HFD-induced increase in body weights (Figure1, Table 2).
Table 2:Influence of RGAP on body weight, food intake, and food efficiency in HFD-fed mice. NC: Normal Control; HFD: High-Fat Diet Group; R300: HFD+RGAP 300mg/kg group; R500: HFD+RGAP500mg/kg group. 1)
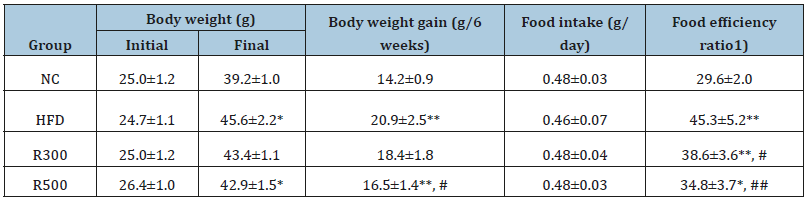
Food efficiency ratio= [Body weight gain(g)/Food intake(g)]
*, ** -significant p<0.05, p<0.01: normal control vs HFD group, R300 or R500 group
#, ## -significant p<0.05, p<0.01: HFD group vs R300 or R500 group
Micro X-ray CT analysis
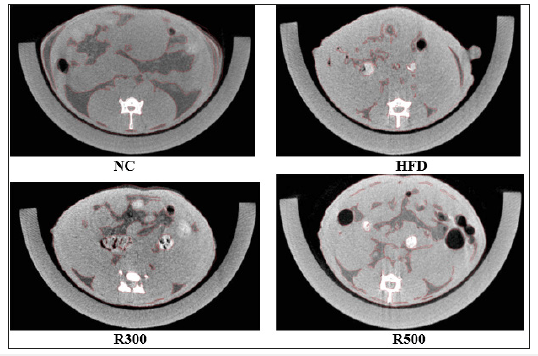
The results of the micro X-ray CT scanning are shown as Figure 2. X-ray CT images showed the positive effect of RGAP on abdominal fat accumulation in mice. The abdominal fat area percent represented in the X-ray CT images was obtained by calculating the area ratio. The abdominal fat mass of X-ray CT in the HFD alone group showed an increase of 18.9% when compared with the NC group. However, HFD plus RGAP 300mg/kg (R300) and HFD plus RGAP 500mg/kg (R500) reduced the increase by 5.4% and 11.1%, respectively, when compared to the HFD group alone.
Figure 2:Micro X-ray CT images of abdominal fat mass in HFD-fed mice. NC: Normal Control; HFD: high-fat diet group; R300: HFD+RGAP300mg/kg group; R500: HFD+RGAP500mg/ kg group
Organ weight, epididymal fat weight and hepatic enzyme
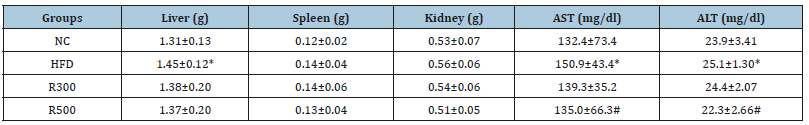
The changes of organ weights, epididymal fat and hepatic enzyme are shown in Table 3. The mice fed the HFD had significantly higher liver weight than that of the mice fed the normal diet (NC). The liver weights in the HFD group increased 10.7% more than that of normal control, but liver weights in the HFD plus RGAP groups (R300, R500) reduced the HFD-induced increase in the weights by 4.8% and 5.5%, respectively. The weights of other organ such as the spleen and kidney did not change between treatment groups. On the other hand, the values of AST and ALT in the HFD group were higher than those in the NC. Administration with RGAP (R300, R500) reduced these values significantly, with p<0.05. These results indicated that HFD feeding in ICR mice for 6 weeks eventually induced obesity parameters such as epididymal fat, liver weight, and hepatic enzymes, and that RGAP effectively prevented HFD-induced obesity. The epididymal fat weight was significantly increased by 18.8% in the HFD group when compared with the NC, but RGAP administration inhibited the epididymal fat deposition, as shown in Figure 3. The epididymal fat weight in the HFD plus RGAP groups (R300, R500) was reduced by 3.6% and 8.5%, respectively, when compared with the HFD group alone.
Figure 3:Effect of RGAP on epididymal fat weight in HFD-fed mice. NC: Normal Control; HFD: High Fat Diet group; R300: HFD+RGAP300mg/kg group; R500: HFD+RGAP500mg/ kg group; *significant p<0.05: NC vs HFD; #significant p<0.05: HFD vs R500
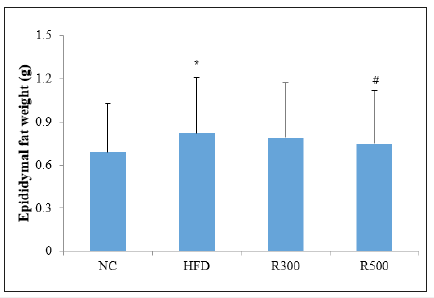
Table 3:Effect of RGAP on organ weight and liver-related enzyme values in HFD-fed mice.
Lipid concentrations
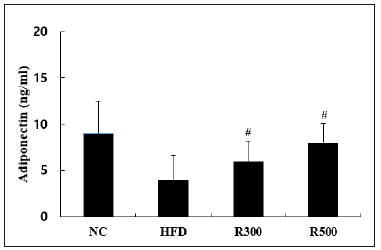
The levels of triglyceride (TG) and LDL in the HFD group were higher than in the NC group, but total cholesterol (TC) and HDL levels were lower. When RGAP was administered, the TG and HDL parameters were recovered, but neither TC nor LDL changed significantly. The TG level in the HFD group was increased 26.0%, but those of the HFD plus RGAP groups (R300, R500) were significantly reduced by 4.5% and 8.3% when compared with the HFD group, respectively. Conversely, the HDL level in the HFD group decreased by 18.8%, but those of the HFD plus RGAP groups (R300, R500) significantly recovered by 15.3% and 16.2%, respectively, when compared with the HFD group. The ratio of LDL/HDL also increased 76.2% in the HFD group, but RGAP administration (R300, R500) reduced this by 16.2% and 42.3%, respectively, when compared with the HFD group (Table 4). RGAP significantly suppressed increases in the TG level and recovered the reduction of HDL. However, RGAP did not alter the levels of TC and LDL. HFD not only elevated serum leptin content but also lowered adiponectin. RGAP administration reversed the rise in leptin and decrease in adiponectin, which are associated with energy expenditure and fatty acid oxidation. The leptin content in the HFD group increased 171.4%, but those of the HFD plus RGAP groups (R300, R500) dropped by 5.3% and 15.8%, respectively, when compared with the HFD group (Figure 4). The adiponectin content in the HFD group decreased 55.6%, but the HFD plus RGAP groups (R300, R500) showed an increase of 50% and 100%, respectively (Figure 5).
Figure 4:Effect of RGAP on serum leptin in HFD-fed mice. NC: Normal Control; HFD: High-Fat Diet; R300: HFD+RGAP300mg/kg; R500: HFD+RGAP500mg/kg; #significant p<0.05: HFD vs R500

Table 4:Effect of RGAP on serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), and lowdensity lipoprotein (LDL) contents in HFD-fed mice.
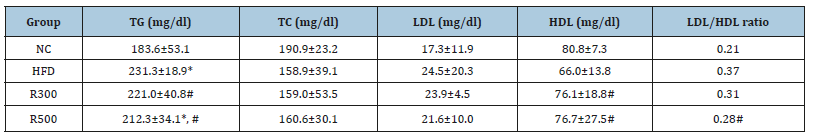
Figure 5:Effect of RGAP on serum adiponectin in HFD-fed mice. NC: Normal Control; HFD: High Fat Diet Group; R300: HFD+RGAP300mg/kg group, R500: HFD+RGAP500mg/ kg group; #significant p<0.05: HFD vs R300 or R500
LPL Activity
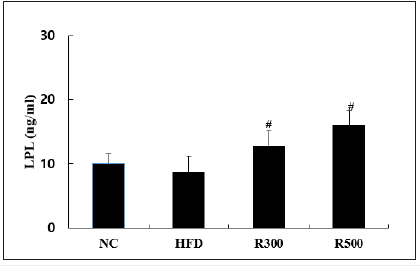
Since it is known that LPL (lipoprotein lipase) is a key atheroprotective enzyme [37] in regulating plasma TG levels by removing TG-rich lipoproteins from circulating plasma [5,38]. We investigated whether RGAP is able to modulate to explain its antiobesity and anti-hyperlipidemic activity with hypoglycemic action due to HFD administration. Interestingly, Figure 6 showed lower LPL activity in the HFD group when compared with the NC group. Orally administered RGAP (R300, R500) dose-dependently upregulated LPL activity by 47.1% and 83.9%, respectively at p<0.05, when compared to the HFD.
Figure 6:Effect of RGAP on serum adiponectin in HFD-fed mice. NC: Normal Control; HFD: High Fat Diet Group; R300: HFD+RGAP300mg/kg group, R500: HFD+RGAP500mg/ kg group; #significant p<0.05: HFD vs R300 or R500
Discussion
We found that RGAP administration reduced the levels of both obesity and hyperlipidemia, indicating that RGAP may diminish the levels of TG through the activation of LPL. Similar results were found with other polysaccharides such as Auricularia auricularderived polysaccharides and fucoidan [38,39]. Therefore, these results suggest that the administration of RGAP in mice may modulate obese conditions by upregulating the degradation enzyme LPL activity. LPL is most abundantly expressed in adipose tissue, macrophages, heart, and skeletal muscle, where it acts as a gatekeeper for the entry of fatty acids into tissue [40-43]. LPL controls systemic lipid partitioning, which is essential for energy homeostasis of the body [44]. Other factors also seem to be involved in the regulation of LPL activity. It has been suggested that the binding between LPL and heparan sulfate proteoglycans plays a critical role in LPL function [40]. However, more research is needed to prove its action mode and another factors. Considering that the higher molecular weight β-glucan is clinically effective in controlling blood lipid composition [41], it is suggested that the anti-obesity activity of RGAP seems to be available in the human body, although its anti-obesity efficacy was seen at higher doses. Whether RGAP can be developed to be functional food with antiobesity properties will be examined in next trial. The analysis of component sugars in RGAP by HPLC revealed that the acidic sugars contained galacturonic acid as a major component and glucuronic acid as a minor component [11]. However, an investigation of which component of RGAP is responsible factor for its anti-obesity effects should be also carried out to reach more detailed pharmacological understanding.
Obesity is defined as abnormal or excessive fat accumulation that may impair health. As a mechanism of fat accumulation, excessive intake of carbohydrates cause fat accumulation by the inhibition of lipolysis and the promotion of lipogenesis from the activation of lipoprotein lipase in adipose tissue. It has been reported that Korean red ginseng extract supplementation to the diet was effective in the suppression of obesity and liver injury induced by chronic HFD feeding [43]. The increase of body weight, epididymal mass, and hepatic lipids was reduced in accordance with the reduction of serum parameters related to obesity. Histological findings and serum hepatic function parameters have indicated that Korean red ginseng extract supplementation protected HFD-fed mice from hepatic injury due to steatosis. This report indicates that the restoration of liver function impaired by the HFD feeding may contribute to the anti-obesity effect of Korean red ginseng extract.
In this study, feeding mice an HFD for 6 weeks resulted in the development of obesity with hepatic injury or dysfunction. HFD-feeding also induced higher abdominal fat deposition and increased body weight. Although food consumption was not changed in the HFD-fed group, the increased caloric density of the HFD led to significantly higher weight gain compared with the normal diet group, resulting in higher food efficiency. Thus HFD-fed mice showed more rapid body weight gain and greater epididymal fat mass than those of mice fed a normal diet. However, the higher body weight gain and food efficiency exhibited in the HFD-fed group were reduced in the RGAP administration groups, which parallels the results obtained by Song et al. [43]. HFD feeding elevated the levels of serum TG and reduced the level of LDL in mice. However, the TG increase was reduced, and the HDL drop was elevated by RGAP administration, indicating that RGAP improves HFD-induced metabolic abnormalities. Micro X-ray CT is a nondestructive technique that allows visualization of the internal structure of mice, determined mainly by variations in density and atomic composition. This is followed by the reconstruction of twodimensional cross-sections perpendicular to the axis of rotation [40]. Findings from our micro X-ray CT images suggest a positive effect of RGAP on abdominal fat accumulation in mice. In this study, we observed that RGAP administration reduced abdominal fat mass in HFD-fed mice. However, more research is needed to elucidate the reducing mechanism.
In previous studies, the activity and level of LPL recovered by RGAP administration in both endogenous and exogenous hyperlipidemic rat models has been demonstrated. TG was mainly decreased by LPL, which is a well-known enzyme that breaks down TG [45]. In this study, increased TG levels were significantly reduced by RGAP administration. Conversely, TC levels were significantly decreased in HFD-fed mice and were not changed by RGAP administration (Table 4). This indicates that RGAP administration did not affect the TC, but influenced TG selectively, which is in line with our previous results [11]. Our previous report has shown that the decrease of serum TG correlates with improved anti-hyperlipidemia in rats, but not with TC. Therefore, the decrease of TG levels associated with RGAP administration may be attributable to the decrease of epididymal fat (Figure 3). HFD-induced obesity, which is associated with abnormal biological metabolism, may be the cause of chronic metabolic diseases [46]. One of them is atherosclerosis, commonly referred to as a hardening or furring of the arteries. Atherosclerosis is induced by the formation of multiple plaques characterized by abnormal lipid metabolism within the arteries [47]. The RGAPinduced improvement in lipid profiles affected by the HFD-including TG, HDL-cholesterol, and LDL-cholesterol-suggests that it may enhance lipid metabolism and prevent obesity. Leptin, a regulator of energy homeostasis in both the central nervous system and the peripheral nerves, is an adipocyte-derived protein which functions as an adipocytokine and also senses and regulates body energy stores [48]. HFD-fed mice increased body weight and plasma leptin concentrations [49]. However, RGAP administration inhibited the rise of leptin concentration. On the other hand, adiponectin also is adipose tissue-specific protein that circulates in human plasma at high levels. It is one of the physiologically active polypeptides secreted by adipose tissue. Rise of adipose has been accompanied by a reduction in plasma glucose and increase in insulin sensitivity. Adiponectin increases insulin sensitivity by increasing tissue fat oxidation, resulting in reduced circulating fatty acid levels and reduced intracellular triglyceride contents in liver and muscle [48]. In the adipocytes of obesity, the expression of adiponectin was shown to be reduced as compared with normal models. Adiponectin depletion stimulates the secretion of free fatty acids on adipocytes. However, in this study, RGAP restored the abnormal or impaired levels of the important indicators-leptin and adiponectinthus improving or delaying HFD-induced metabolic abnormalities. These results demonstrate that RGAP regulates imbalances of leptin and adiponectin, which result in metabolic disorder induced by HFD. Thereby, it indicates that RGAP can effectively improve HFD-induced impairments in obesity.
In this study, RGAP manifested dose-dependent effects on increases in body weight, epididymal fat weight, abdominal fat mass, and impaired serum biochemical parameters under HFD conditions. According to these results, we can conclude that RGAP regulates HFD-induced abnormal lipid metabolism via the modulation of the levels of leptin and adiponectin, thereby resulting in an antiobesity effect. In conclusion, we found that RGAP recovered the levels leptin, adiponectin, and especially the level of TG through activation of LPL, suggesting that RGAP may improve obese-related conditions in metabolic syndromes. In future studies, the active components of RGAP obtained by molecular fractionation as well as the mechanisms relevant to the anti-obesity effects of RGAP in relation to lipid metabolism will be evaluated.
Conclusion
It can be concluded that red ginseng acidic polysaccharide from Korean red ginseng protected against both obesity and hyperlipidemia in high-fat diet mice models. Red ginseng acidic polysaccharide exerted anti-obesity effects in the animal models fed an HFD via the activation of lipoprotein lipase and the improvement of leptin and adiponectin, which carry out critical functions in energy and lipid metabolism. These results suggest that red ginseng acidic polysaccharide might be a preventative functional food for these metabolic disorders.
References
- Lee SM, Bae BS, Park HW, Ahn NG, Cho BG, et al. (2015) Characterization of Korean red ginseng (Panax ginseng Meyer): History, preparation method and chemical composition. J Ginseng Res 39(4): 384-391.
- Brekhman II, Dardymov IV (1969) New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol 9: 419-430.
- Kim YJ, Lee OR, Lee SY, Kim KT, Yang DC (2012) Isolation and characterization of a theta glutathione S-transferase gene from Panax ginseng C.A. Meyer. J Ginseng Res 36(4): 449-460.
- Liu CX, Xiao PG (1992) Recent advances on ginseng research in China. J Ethnopharmacol 36(1): 27-38.
- Wang J, Li SS, Fan YY, Chen Y, Liu D, et al. (2010) Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer. J Ethnopharmacol 130(2): 421-430.
- Babiker LB, Gadkariem EA, Alashban RM, Assihar HI (2014) Investigation of stability of Korean ginseng in herbal drug product. Am J Appl Sci 11(1): 160-170.
- Zhang DT, Yasuda YY, Zheng P, Kawabata T (1996) Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med 20(1): 145-150.
- Yun TK, Choi SY, Yun HY (2001) Epidemiological study on cancer prevention by ginseng: Are all kinds of cancers preventable by ginseng? J Korean Med Sci S19-S27.
- Joo SS, Won TJ, Lee I (2005) Reciprocal activity of ginsenosides in the production of proinflammatory repertoire and their potential roles in neuroprotection in vivo. Planta Med 71(5): 476-481.
- Jung CH, Seog HM, Choi IW, Choi HD, Cho HY (2005) Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol 98(5): 245-250.
- Kwak YS, Kyung JS, Kim JS, Cho JY, Rhee MH (2010) Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull 33(3): 468-472.
- Oh J, Lee H, Park D, Ahn J, Shin SS, et al. (2012) Ginseng and its active components ginsenosides inhibit adipogenesis in 3T3-L1 cells by regulating MMP-2 and MMP-9. Evid Based Complement Alternat Med 2012: 265023.
- Song MY, Kim BS, Kim H (2014) Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J Ginseng Res 38(2): 106-115.
- Blumenthal M (2001) Asian ginseng: potential therapeutic uses. Adv Nurse Pract 9(2): 26-28.
- Choi KT (2008) Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C.A. Meyer. Acta Pharmacol Sin 29(9): 1109-1118.
- Park TY, Park MH, Shin WC, Rhee MH, Seo DW, et al. (2008) Antimetastatic potential of ginsenoside Rp1, a novel ginsenoside derivative. Biol Pharm Bull 31(9): 1802-1805.
- Konno C, Sugiyama K, Kano M, Takahashi M, Hikino H (1984) Isolation and hypoglycemic activity of Panaxans A, B, C, D and E, glycans of Panax ginseng roots. Planta Med 50(5): 434-436.
- Oshima Y, Konno C, Hikino H (1985) Isolation and hypoglycemic activity of Panaxans I, J, K and L, glycans of Panax ginseng roots. J Ethnopharmacol 14(2-3): 255-259.
- Konno C, Murakami M, Oshima Y, Hikino H (1985) Isolation and hypoglycemic activity of Panaxans Q, R, S, T and U, glycans of Panax ginseng roots. J Ethnopharmacol 14(1): 69-74.
- Tomoda M, Shimada K, Konno C, Sugiyama K, Hikino H (1984) Partial structure of Panaxan A, a hypoglycemic glycan of Panax ginseng roots. Planta Med 50(5): 436-438.
- Gao QP, Kiyohara H, Cyong JC, Yamamda H (1991) Chemical properties and anti-complementary activities of heteroglycans from the leaves of Panax ginseng. Planta Med 57(2) 132-136.
- Park KM, Kim YS, Jeong TC, Joe CO, Shin HJ, et al. (2001) Nitric oxide is involved in the immunomodulating activities of acidic polysaccharide from Panax ginseng. Planta Med 67(2): 122-126.
- Du XF, Jiang CZ, Wu CF, Won EK, Choung SY (2008) Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch Pharm Res 31(9): 1153-1159.
- Okuda M, Lee SD, Matsuura Y, Zheng Y, Sekiya K, et al. (1990) Biological activities of non-saponin compounds isolated from Korean red ginseng. Proceeding of Int Symp on Korean Ginseng. Seoul, Korea, pp. 15-19.
- James PT, Rigby N, Leach R (2004) The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Pre Rehabil 11: 3-8.
- Yamamoto M, Uemura T, Nakama S, Uemiya M, Kuumagai A (1983) Serum HDL-cholesterol increasing and fatty liver improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man. Am J Chin Med 11(1-4): 96-101.
- Qureshi AA, Abuirmeileh N, Din ZZ, Ahmad Y, Burge WC, et al. (1983) Suppression of cholesterogenesis and reduction of LDL cholesterol by dietary ginseng and its fractions in chicken liver. Atherosclerosis 48(1): 81-94.
- Kim SH, Park KS (2003) Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res 48(5): 511-513.
- Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ, et al. (2007) Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun 364(4): 1002-1008.
- Karu N, Reifen R, Kerem Z (2007) Weight gain reduction in mice fed Panax ginseng saponin, a pancreatic lipase inhibitor. J Agric Food Chem 55(8): 2824-2828.
- Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, et al. (2005) Effect of crude saponin on Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci 97(1): 124-131.
- Inoue M, Wu CZ, Dou DQ, Chen YJ, Ogihara Y (1999) Lipoprotein lipase activation by red ginseng saponins in hyperlipidemia model animals. Phytomedicine 6(4): 257-265.
- Hwang JT, Lee MS, Kim HJ, Sung MJ, Kim MS, et al. (2009) Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother Res 23(2): 262-266.
- Kim JH, Kang SA, Han SM, Shim I (2009) Comparison of the antiobesity effect of the protopanaxadiol and protopanaxatriol-type saponins of red ginseng. Phytother Res 23(1): 78-85.
- Shin HJ, Kim YS, Kwak YS, Song YB, Kim YS, et al. (2004) Enhancement of antitumor effects of Paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP). Planta Med 70(11): 1033-1038.
- Van Geet M, Swennen R, David P (2001) Quantitative coal characterization by means of microfocus X-ray computer tomography, colour image analysis and back scatter scanning electron microscopy. Int J Coal Geol 46(1): 11-25.
- Chiba T, Miura S, Sawamura F, Uetsuka R, Tomita I, et al. (1997) Antiatherogenic effects of a novel lipoprotein lipase-enhancing agent in cholesterol-fed New Zealand white rabbits. Arterioscler Thromb Vasc Biol 17(11): 2601-2608.
- Yokota T, Nagashima M, Ghazizadeh M, Kawanami O (2009) Increased effect of fucoidan on lipoprotein lipase secretion in adipocytes. Life Sci 84(15-16): 523-529.
- Chen G, Luo YC, Li BP, Li B, Guo Y, et al. (2008) Effect of polysaccharide from Auricularia auricula on blood lipid metabolism and lipoprotein lipase activity of ICR mice fed a cholesterol-enriched diet. J Food Sci 73(6): H103-H108.
- Spillmann D, Lookene A, Olivecrona G (2006) Isolation and characterization of low sulfate heparan sulfate sequences with affinity for lipoprotein lipase. J Biol Chem 281(33): 23405-23413.
- Keenan JM, Goulson M, Shamliyan T, Knutson N, Kolberg L, et al. (2007) The effects of concentrated barley beta-glucan on blood lipids in a population of hypercholesterolaemic men and women. Br J Nutr 97(6): 1162-1168.
- Lloyd E. E, Gaubatz JW, Burns AR, Pownall HJ (2007) Sustained elevations in NEFA induce cyclooxigenase-2 activity and potentiate THP- 1 macrophage foam cell formation. Atherosclerosis 192(1): 49-55.
- Song YB, An YR, Kim SJ, Park HW, Jung JW, et al. (2012) Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J Sci Food Agric 92(2): 388-396.
- Williams RR, Hunt SC, Hopkins PN, Wu LL, Lalouel JM (1994) Evidence for single gene contributions to hypertension and lipid disturbances: definition, genetics and clinical significance. Clin Genet 46(1): 80-87.
- In G, Ahn NG, Bae BS, Lee MW, Park HW, et al. (2017) In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng and red ginseng. J Ginseng Res 41(3): 361-369.
- Laclaustra M, Corella D, Ordovas J (2007) Metabolic syndrome pathophysiology: The role of adipose tissue. Nutr Metab Cardiovasc Dis 17(2): 125-139.
- Morrison CD, Huypens P, Stewart LK, Gettys TW (2009) Implication of crosstalk between leptin and insulin signaling during the development of diet-induced obesity. Biochim Biophys Acta 1792(5): 409-416.
- Diez JJ, Glesias P (2003) The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 148(3): 293-300.
- Venancio JC, Marqatho LO, Rorato R, Rosales RRC, Debarba LK, et al. (2017) Short-term high-fat diet increases leptin activation of CART neurons and advances puberty in female mice. Endocrinology 158(11): 3929-3942.
© 2019 Yi-Seong Kwak. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






