- Submissions

Full Text
Advances in Complementary & Alternative medicine
Acute and 28 days Toxicity Assessment of Aqueous Extract of Stem Back of Musanga Cecropioides (Urticaceae)
Medou FM, Nyunaï N*, Bika Lele EC, Oumarou GH and Metsadjio AN
Institute of Medical Research and Medicinal Plants Studies (IMPM), Cameroon
*Corresponding author:Nyunaї Nyemb, Medical Research Centre, Institute of Medical Research and Medicinal Plants Studies (IMPM), Yaoundé, Cameroon
Submission: May 08, 2019Published: May 16, 2019

ISSN: 2637-7802 Volume4 Issue2
Abstract
Musanga cecropioides is a plant widely used in many countries in Africa for the treatment of various diseases. However, few studies have been carried out to evaluate the toxicities of the different parts of this plant. This study aims to investigate the acute and the subacute toxicity of the aqueous extract obtained from the stem bark of Musanga cecropioides. The acute toxicity of M. cecropioides Aqueous Extract (MCAE) were first evaluated on mice to determine the DL50. Then 4 groups of male and female rats were subjected to subacute toxicity for 28 days, with daily oral administration of 200, 300 and 600mg/kg of aqueous extract. A fourth group of male and female rats serving as control took only distilled water. Body weight was monitored at the beginning, and weekly. On the 29 day the animals were sacrificed and then liver, kidney, heart, lungs, spleen, pancreas, testis and ovaries were removed for morphometric and histological examinations. The weight and blood were serving to evaluate hematological parameters, while serum was utilized to determine ALAT, ASAT, total protein, urea, lipid profile (Total Cholesterol, triglycerides, HDL Cholesterol and LDL Cholesterol). The results show that MCAE has an LD50 of over 2000mg/kg. In the subacute toxicity the weight gain between groups at the beginning and at the end did not show any significant difference. Relative organ weight was not affected by any treatment at doses used. The lipid profile, ASAT, ALAT, serum urea, total protein in treated groups did not show any important difference when compared with the control group in males and in females. Hematological results showed significant increase in hematocrit in males treated rats (p˂0.05) at 200mg/kg and reduce in platelets in female rats treated with 300mg/kg (p˂0.05). Histologically, neither gross abnormalities nor sign and symptoms of degeneration on the isolated organs at the end of study were seen. No mortality was recorded for the 28 days. These results reveal the safety of oral administration of the stem bark extract of Musanga cecropioides. Moreover, this plant could also have protective effects on the liver.
Keywords:Musanga cecropioides; Toxicity; Bark; Aqueous Extract; Rat; Mice
Abbreviations: MCAE: M.cecropioides Aqueous Extract; WBC: White Blood Cells; RBC: Red Blood Cells; PLT: Thrombocyte Count; HGB: Hemoglobin; HCT: Hematocrit; MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Hemoglobin; MCHC: Mean Corpuscular Hemoglobin Concentration; BUN: Blood Urea Nitrogen; Glu: Blood Glucose; TP: Total Protein; TG: Triglyceride ; TC: Total Cholesterol; HDL: High Density Cholesterol; LDL: LDL Cholesterol; AI: Atherogenic Index; ALAT: Alanine Transaminase; AST: Aspartate Transaminase; BW: Body Weight; LD50: Median Lethal Dose
Introduction
Musanga cecropioides R.Br. ex Tedlie (Cecropiaceae) is a therapeutic plant generally utilized in Cameroon, Gabon, Nigeria and therefore, the Democratic Republic of Congo in the treatment of numerous diseases, among that are cough, constipation, schizophrenia, chest infection, rheumatism, leprosy, trypanosomiasis, high blood pressure, toothache, malaria, wounds and jaundice [1-3]. Some scientific works have shown the effects of its extracts against hypotension, hyperglycemia, diabetes, diarrhea and bacteria [3,4-6]. Available literature reports the scientifically established uterotonic effects of the leaf in rats [7], the water extracts from the leaf and stem bark exhibited hypotensive effects [8-10]. The plant is known to have potent allelopathic and herbicidal activities [11]. Various parts of M. cecropioides are used in folk medicine for curing sexual transmitted diseases, broncho-pulmonary affections, leprosy, high blood pressure, chest troubles, and many other pathologic disorders [12-14].
The bark-macerate is employed as a decoction for pulmonic disorders, while the bark scrapings are used as blood purifier, galactogogue, analgesic and antipyretics [15]. The root sap is employed as a topical lotion for pulmonary congestion, as lactation stimulants, as pain killer and among the treatment of rheumatism, blood disorders and venereal diseases [15,10]. Many chemical compounds have already been isolated from all parts of this plant. Some triterpenoid acids like kalaic, musangic and cecropioic acid, and a host of other alternative organic chemistry compounds were identified in many studies [16-24]. Daily oral dosing of 750mg/kg body weight of crude aqueous stem bark extract of Musanga cecropioides for 28 days showed that administration of Musanga cecropioides is safe [25]. Following fourteen days investigation of the acute toxicity and 28 days for subacute toxicity on evaluating a combination of the aqueous extracts of the trunk bark of Musanga cecropioides and the fruits of Combretum micranthum, led to no abnormality of the studied parameters in general, while variation of most parameters in amongst groups during the subacute toxicity study was noticed [26].
The hepatoprotective impact of hydromethanolic leaf extract of Musanga cecropioides (MCHL) on carbon tetrachlorideinduced liver damage and oxidative stress demonstrated that Musanga cecropioides extract has a dose-specific hepatoprotective effect; thus, using this extract for the management of liver disease needs alert [27]. In addition, Adeneye [28] showed that the hepatoprotective activities and the mechanisms of actions of Musanga cecropioides stem bark aqueous extract (MCW) investigated on acute hepatocellular injuries induced by intraperitoneal carbon tetrachloride involved flavonoids and alkaloids in high concentrations in MCW. Few studies have been done to assess the safety of the watery extract of Musanga cecropioides, and its effect on certain key parameters, especially transaminases and the histology of detoxifying organs. The point of this investigation was to evaluate the acute and subacute toxicity of the aqueous stem bark extract of Musanga cecropioides utilizing 3 increasing doses up to 600mg/kg for 28 days.
Materials and Methods
Plant material collection and extraction
Fresh M. cecropioides stem barks were gathered at Mbankomo, Yaoundé suburbs (Centre Region, Cameroon) in February 2018. Bark pieces were dried under room temperature and ground into powder with an electrical blender. 225g of the powder was introduced into 4L of distilled water and stewed for 20 minutes. The subsequent decoction was separated through Whatman paper NO. 3 and further lyophilized. A purple concentrate powder (M. cecropioides Aqueous Extract (MCAE), 14.625g) was obtained, giving a yield of 6.5%.
Acute toxicity of the aqueous extract of Musanga cecropioides in female mice.
Female Balb C mice, with weight between 18g and 22g, were utilized for the acute toxicity study. A number of 6 female mice were used to assess the acute toxicity of the MCAE. Using an oral feeding needle, the extract solution was initial administered at a single dose of 2000mg/kg to 3 mice previously fasted for 12h. The behavior of the animals was ascertained for 30 minutes when this administration, and so they were fed. If no deaths were seen following 48 hours, the identical protocol was continued on 3 alternative female mice. All the six mice were watched for 14 days. Throughout this period, animal weight was recorded every two days, and rats were sacrificed on day 14 for macro-observations of internal organs [29].
28 days toxicity evaluation of the aqueous extract of Musanga cecropioides in rats.
Experimental animals: Wistar rats (125-190g) (20 males and 20 females) were reared at the Institute of Medical Research and Medicinal Plants studies (IMPM) of Yaoundé, Cameroon. They were kept at normal laboratory conditions under natural light and dark cycles, at consistent room temperature (20±5 °C) and were freely allowed to possess food and water.
Ethical consideration: Ethical approval was given by the Institutional Review Board (IRB) of the Institute of Medical Research and Medicinal Plants Studies and administrative authorization was obtained to conduct this study within the animal house of this Institution.
28 days subacute toxicity experiments
Animals (20 males and 20 females) were fairly divided into 4 groups (5 animals/dose/sex). The first group was given 1mL/100g b.w distilled water and brought as a control. The second, third and fourth groups were given a dose of 200, 300, and 600mg/ kg of M. cecropioides Aqueous Extract (MCAE) respectively, by feeding using an oral needle daily, for a period of 28 days. Body weight was checked week after week. Towards the finish of the exploratory period, all animals were fasted for 12h. Thenceforth they were anesthetized by intraperitoneal infusion of diazepam/ ketamine (10/50mg/kg). Blood samples were gotten from the arterial blood vessel for hematological analysis and serum organic chemistry. The animal abdomen was opened by thoracic abdominal longitudinal incision and the inner organs (liver, kidneys, heart, lungs, spleen, pancreas and testis or ovaries) were detached and weighed to estimate the relative organ weights (using the formula):
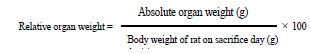
Histopathological examination
After macroscopic analysis, representative fragments of liver, left kidney, heart, spleen and lungs were fixed in a 10 % solution of buffered formalin (pH 7.4) and enclosed in paraffin, following technique of Mayer [30] with slight modifications and then observed under a light microscope. Five-micrometer sections were gotten and colored with Hematoxylin-Eosin (HE) for analysis under an optical microscope.
Hematology and serum biochemistry
Hematological examination was carried out on blood samples collected in EDTA-coated tubes. White Blood Cells (WBC), Red Blood Cells (RBC), Trombocyte count (PLT), hemoglobin (HGB), hematocrit (HCT), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC) were measured using the Coulter Counter System (Beckman Coulter®, ThermoFisher, UK).
The blood samples without decoagulant were centrifuged at 3000 RPM at 4 °C for 10 minutes, at that point the serum for biochemical examination was isolated. Blood urea nitrogen (BUN) and blood glucose (Glu) were estimated utilizing commercial kits obtained from Biotec Diagnostics UK LTD, while Total Protein (TP), Triglyceride (TG), Total Cholesterol (TC), High Density Cholesterol (HDL) alanine transaminase (ALT) and aspartate transaminase (AST) were estimated utilizing commercial kits got from SGM Italia, Italy.
Data Analysis
The results were reported as mean±SEM. The statistical significance was determined by using one-way analysis of variance (ANOVA) followed up by Dunnett’s multiple comparison posthoc test. P values less than 0.05 were considered as significant. GraphPad Prism version 5.03 was utilized for all measurable data.
Results
Effect of Musanga cecropioides aqueous extract on body weight of male and female rats
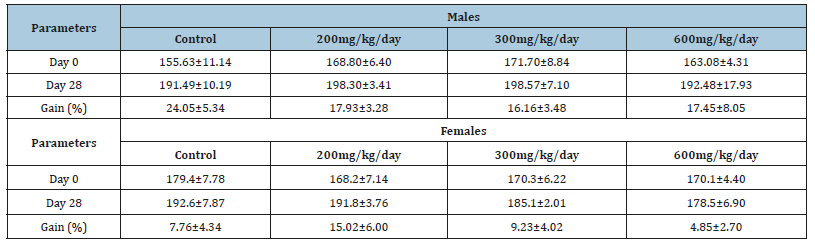
The administration of the aqueous extract showed an identical weight gain in the 3 groups of male rats treated with the extract in comparison to the control, despite a non-significant high weight gain observed in the control group (24.05±5.34 to 17.93±3.28, at 16.16±3.48, at 17.45±8.05 for respectively 200mg/kg, 300mg/kg, and 600mg/kg) (Table 1). In females rats the body weight gain appears to be lower in the different groups (when compared to males). However, it remains the same in all the treated groups as compared to control (Table 1)
Table 1:Body weight changes of male and female rats given Musanga cecropioides orally for 28 days. Values are mean±SEM. N=5.
Effect of Musanga cecropioides aqueous extract on relative organ weight of male and female rats
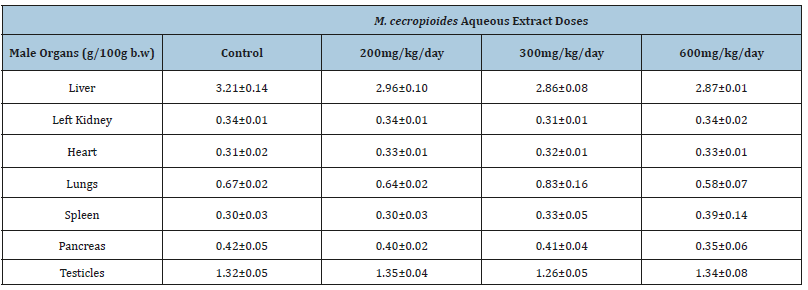
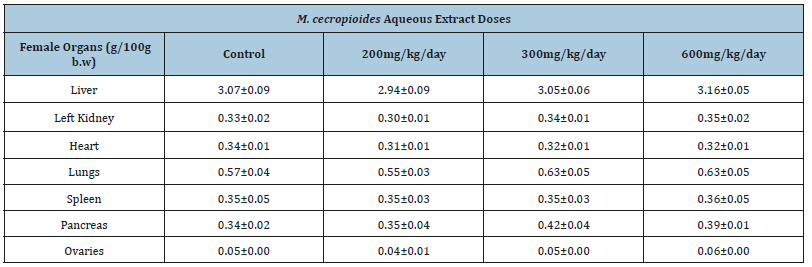
The relative tissue weights were not altered by Musanga cecropioides treatment. The weight of the various organs (liver, kidney, heart, lungs, spleen, pancreas, testes and ovaries) related to body weight of the animals did not show any significant differences, either in male or female rats (Table 2A & Table 2B).
Table 2A:Effect of the 28-day subacute toxicity of the aqueous extract of Musanga cecropioides on the relative organ weights of male rats. Values are mean±SEM.
N: 5; bw: body weight
Table 2B:Effect of the 28-day subacute toxicity of the aqueous extract of Musanga cecropioides on the relative organ weights of female rats. Values are mean±SEM.
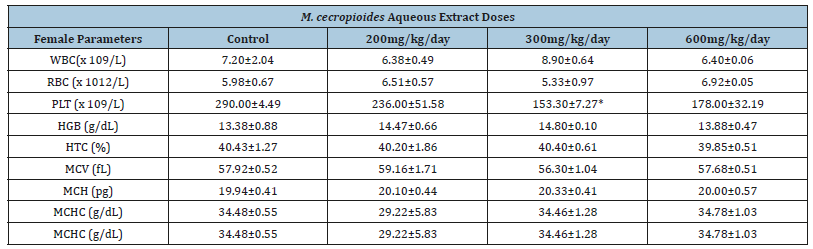
Effect of Musanga cecropioides aqueous extract on biochemical markers of male and female rats
AST, ALT, total protein, urea, and glucose are the biochemical markers measured in male and female rats. No significant differences were noted for these different parameters for either male rats or female rats. However, the total protein content fluctuated in both sexes despite a non-significant difference for both the observed values in males and for the values observed in females (Table 3).
Table 3:Effects of the aqueous extract of Musanga cecropioides on liver and kidney markers. Values are mean±ESM. N = 5.
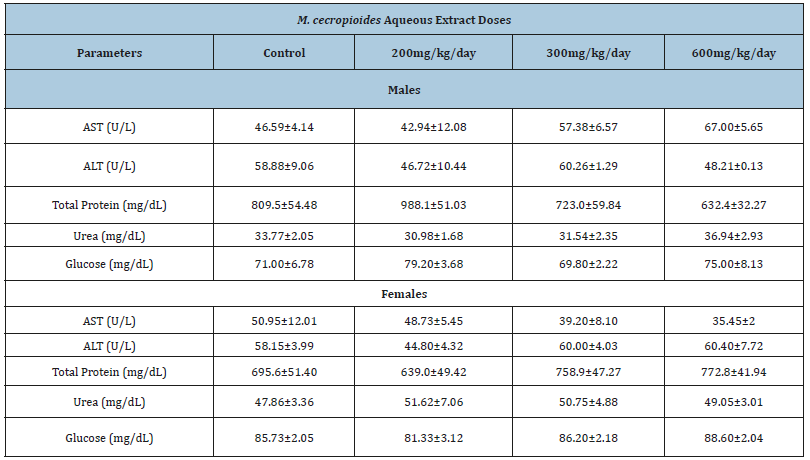
Effect of Musanga cecropioides aqueous extract on the hematological parameters of male and female rats
At the end of the experiment, the hematological analysis revealed a lack of significant difference between the value of hematological parameters in the treated groups when compared to the control group in both sexes, except for the males where there was an increase in hematocrit (37.26±2.10 to 43.04±0.93) at a dose of 200mg/kg (p˂0.05) (Table 4A) and in females where platelets were decreased (290.00±4.49 to 153.30±7.27) at a dose of 300mg/kg (p˂0.05) (Table 4B).
Table 4A:Effect of the 28-day subacute toxicity of the aqueous extract of Musanga cecropioides on the hematological parameters in rats. Values are mean±SEM. N = 5. *p ˂ 0.05 significant difference from the control.

Table 4B:Effect of the 28-day subacute toxicity of the aqueous extract of Musanga cecropioides on the hematological parameters in rats. Values are mean±SEM. N=5. *p ˂ 0.05 significant difference from the control.
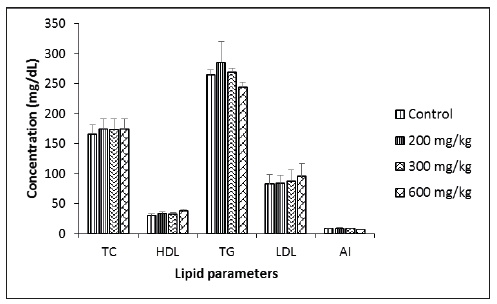
Effect of Musanga cecropioides aqueous extract on Lipid profile of male and female rats
At the end of the experiment the serum lipid profile of the male rats (Figure 1) and that of the females (Figure 2) was evaluated. No significant differences were found for total Cholesterol, HDL Cholesterol, triglycerides, nor for LDL Cholesterol between the treated groups in comparison to the control group. The atherogenic index remained equally identical in all groups of animals for both sexes.
Figure 1:Lipid profile of male rats. TC: Total Cholesterol; HDL: HDL Cholesterol; TG: Triglycerides; LDL: LDL Cholesterol; AI: Atherogenic Index; LDL=([TC-HDL] -([TG]/5)); AI=(TC-HDL)/HDL. Each bar represents mean±SEM; N=5.
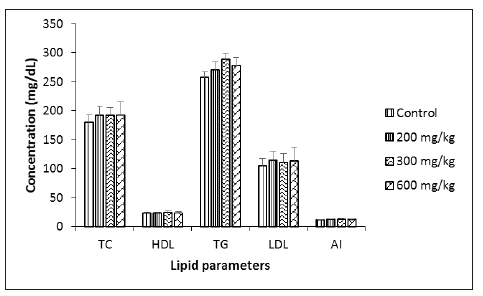
Figure 2:lipid profile of female rats. TC: Total Cholesterol; HDL: HDL Cholesterol; TG: Triglycerides; LDL: LDL Cholesterol; AI: Atherogenic Index. LDL=([TC-HDL] -([TG]/5)); AI=(TC-HDL)/HDL. Each bar represents mean±SEM; N=5.
Histopathology
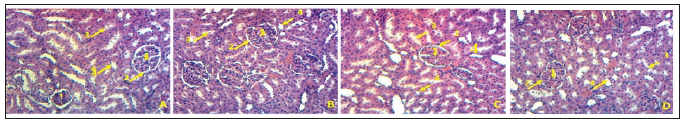
The macroscopic analysis of the target organs of the treated animals did not show significant changes in color and texture when compared with the control group. In addition, the microscopic analysis did not show histological alterations in liver or in kidney, even in heart, lungs and spleen (data not shown). The histological sections of the liver of rats in the control group, as well as the rats receiving the extract at different doses, show a normal architecture of the hepatic parenchyma with a portal space (portal vein, biliary duct and hepatic artery), hepatocytes and distinct sinusoids (Figure 3). Histological analysis of the kidneys of normal control rats and those of animals treated at different doses revealed no significant renal parenchyma abnormalities (Figure 4).
Figure 3:Photomicrograph of the liver of rats treated with the extract of Musanga cecropioides (magnification-100): (A) Control; (B) 200mg/kg; (C) 300mg/kg; (D) 600mg/kg. 1- portal vein; 2 -biliary duct; 3 -hepatic artery; 4 -Hepatocyte; 5 -Sinusoids.
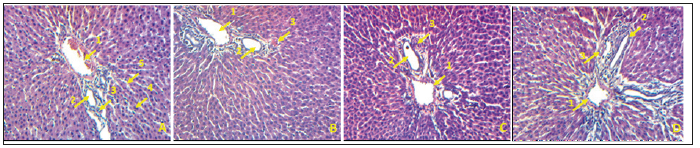
Figure 4:Photomicrograph of the kidney of rats treated with the extract ofMusanga cecropioides (magnification100): (A) Control; (B) 200mg/kg; (C) 300mg/kg; (D) 600mg/kg. 1 -Glomerulus; 2 -Urinary pole; 3 -Proximal convoluted tubule; 4 -Distal Convoluted tubule.
Discussion
Traditional medicine uses plant taking only in account their biological activities. Musanga cecropioides is extensively used in Africa for the treatments of several ailments. Therefore, this study was focused on evaluation of acute and subacute toxicity of Musanga cercropioides aqueous extract. The acute toxicity study exhibited no death at 2000mg/kg dose of aqueous extract administered to female rats. So, this aqueous extract of Musanga cecropioides can be considered to be lower toxic substances, since its LD50 was greater than 2000mg/kg [29]. The result is similar to the one of Adeneye et al. [25] who estimated the LD50 of the stem bark aqueous extract of Musanga cecropioides to be greater than 3000mg/kg b.w/oral route.
In subacute studies, male and female rats were checked during 28 days in daily administration of 200mg/kg, 300mg/kg and 600mg/kg. During the experiment, body weight, organ weight, hematological, biochemical parameters as well as histology of organs were set in both sexes. Animal weight is one of the criteria used for estimating the medical condition of experimental animals [31]. Exposure to toxic substances, will induce a variation in body weight gain, and internal organ weights which would reflect toxicity [31]. Weight loss is also associated with adaptation reaction following exposition of body to plant extract [32]. Plant extracts can also cause appetite suppression, producing weight loss. [31]. Throughout this study, regardless of the doses used (200, 300 and 600mg/kg of body weight), Musanga cecropioides extract did not appear to affect the body weight of the rats either in males or females (p>0.05) between control and all treated groups. These results are different to that of Adeneye et al. [25]; they evoked a reduction of the weight of the animals (P<0.05), after treating rats with 750mg/kg of the stem bark aqueous extract of Musanga cecropioides for 4 weeks. Another work done by Legbosi & Ellis. [33] on hydromethanolic stem bark extract of M. cecropioides in subchronic study at doses of 125, 250 and 500mg/kg for 96 days, reported increase of weight of both sexes of rats.
Organ weight allows to know the state of health, to know if the organ is altered or not, with hypertrophy or hypotrophy [34-36]. The main site of metabolic reactions of poisons are the liver, heart, lungs, kidneys and spleen [36]. The relative ratio of organ to body weight in this study showed no significant differences in treated groups (p>0.05) when compared to control, suggesting that the extract did not appear to affect the rat’s organs. It can be suggested that M. cecropiodes extract is practically nontoxic, in accordance with these results of Adeneye et al. [25].
The liver is the key organ of metabolism, ALT and AST are enzymes that reflect its activity, its alteration or its degradation [37]. Emerson et al. [38] also have recorded that increasing in serum protein is indicative of tissue destruction and reflects liver toxicity. No significant difference in the levels of total proteins, ALT and AST was found. Micrographies of liver at all doses used showed normal structure, correlated with normal values of ALT and AST, suggests that the extract may not be toxic to the liver and could equally have hepatoprotective effects. Nwidu et al. [27] raised this possibility after observing regeneration of liver of intoxicated rats with CCl4 and treated with 141.4mg/kg of M. cecropioides hydromethanolic leaf extract. Similarly, Omoruyi et al. [39] found the same result on carbon tetrachloride-induced non-alcoholic fatty liver disease in Wistar rats. Serum urea is the indicator of good drug reabsorption and excretion, but also useful to evaluate kidney function especially renal sufficiency [40,41]. Thus, higher levels of serum urea are reflection of renal dysfunction. In present study, there were no significant changes in blood urea. These results with histological observations indicate that the kidney tasks were not affected in animals treated with the extract in both sexes. The level of serum glucose was also evaluated. Since in all groups tested the value was not modified in relation to control, we can conclude that M. cecropiodes stem-bark aqueous extract (MCAE) does not affect carbohydrate metabolism.
Hyperlipidemia, especially the elevation of LDL Cholesterol and triglycerides are factors of exposure to cardiovascular diseases, in contrast to a high level of HDL that protects [42-44]. The exposure of the rats to MCAE stem-bark extract for 28 days did not provoke any significant alteration of Total Cholesterol, HDL Cholesterol, LDL Cholesterol and Triglycerides when compared to the untreated control. These results concord with those of Adeneye et al. [25] who after 4 weeks of the crude aqueous stem bark extract of Musanga cecropioides (MCW) treatment at 750mg/kg did not find any significant differences between total protein, Cholesterol, serum urea, ALT and AST transaminases. However, the work of Dibong et al. [26] show, on the contrary, that the mixture (50% of each extract of the used dose) of aqueous extract of the trunk bark of Musanga cecropioides and the fruits of Combretum micranthum administered to the animals for 28 days at doses of 171mg/kg and 1000mg/ kg respectively showed a significant variation in the glycemia, transaminases, HDL Cholesterol, triglycerides and total Cholesterol. This suggests that these side effects observed may be due to the Combretum micranthum extract and not to the Musanga cecropioides extract. Furthermore, Legbosi & Ellis. [33] found that there were no significant effects on lipid profile, after 96 days of treatment with hydromethanolic stem bark extract of Musanga cecropioides. These differences in results obtained by different authors may also be due to the composition of different extracts used, because the quantity and the quality of secondary metabolites of a plant depends on the environmental conditions (soil, climate, etc ...) [45].
Hematology is a very important examination that gives general information about the state of health and immune response of the body [46,47]. In this study significant increase in hematocrit in male treated rats (p˂0.05) at 200mg/kg and decrease in PLT in female animals treated with 300mg/kg (p˂0.05) was observed. These 2 results, despite their significant differences in comparison to the control, can be considered isolated, since at the higher doses of extracts, no significant difference was observed. Therefore, this may not be due to the unsafe outcome of repeated administration of the plant extract. This may lead to the explanation that Musanga cecropioides did not affect hematopoietic system, at the doses used. In addition, Legbosi & Ellis. [33] established also no significant effects of hydromethanolic stem bark extract of Musanga cecropioides on hematology in a sub-chronic study utilizing doses of 125, 250 and 500mg/kg for 96 days.
In addition to hematology, histology can explain the results obtained in biochemistry [29]. Histology allows direct visualization of cell and tissue damage [48]. Treatment of animals of both sexes with Musanga cecropioides extract, at different doses of 200, 300 and 600mg/kg did not induce hepatic, renal, pulmonary, cardiac or even splenic damage.
Conclusion
This study showed that the use of the Musanga cecropioides stem bark aqueous extract, at high doses does not present any danger on the morphometric, biochemical or even histological parameters. Several of these observations lead to the conclusion that liver, kidney, lungs, heart, and spleen are not target organs for both sexes at the doses used and would justify the empirical use of this plant in the treatment of various diseases.
Acknowledgment
The authors are thankful to The World Academy of Sciences (TWAS), for the research grant N° TWAS Research Grant Award_17-432 RG_BIO_AF awarded to Dr.Nyunai Nyemb.
References
- Akendengué B (1992) Medicinal plants used by the Fang traditional healers in Equatorial Guinea. J Ethnopharmacol 37(2): 165-173.
- Akendengué B, Louis AM (1994) Medicinal plants used by the Masango people in Gabon. J Ethnopharmacol 41(3): 193-200.
- Fomogne FMCY, Van VS, Ndinteh DT, Krause RWM, Olivier DK (2014) Antibacterial activities of plants from Central Africa used traditionally by the bakola pygmies for treating respiratory and tuberculosis-related symptoms. J Ethnopharmacol 155(1): 123-131.
- Adeneye AA, Ajagbonna OP, Ayodele OW (2007) Hypoglycemic and antidiabetic activities of the stem bark aqueous and ethanol extracts of Musanga cecropioides in normal and alloxan-induced diabetic rats. Fitoterapia 78(7-8): 502-505.
- Owolabi OJ, Ayinde BA, Nworgu ZA, Ogbonna OO (2010) Antidiarrheal evaluation of the ethanol extract of Musanga cecropioides stem bark. Methods Find Exp Clin Pharmacol 32(6): 407-411.
- Ayinde BA, Omogbai EKI, Onwukaeme DN (2010) Hypotensive effects of 3, 4-dihydroxybenzyaldehyde isolated from the stem bark of Musanga cecropioides. Journal of Pharmacognosy and Phytotherapy 1(1): 4-9.
- Kamanyi A, Bopelet M, Tatchum TR (1992) Contractile effect of some extracts from the leaves of Musanga cecropioides (cecropiaceae) on uterine smooth muscle of the rat. Phytotherapy Research 6(3): 165-167.
- Kamanyi A, Bopelet M, Aloamaka CP, Obeifuna PCM, Ebeigbe AB (1991) Endothelium-dependent rat aortic relaxation to the aqueous leaf extract of Musanga cecropioides. J Ethnopharmacrol 34(2-3): 283- 286.
- Dongmo AB, Kamanyi A, Bopelet M (1996) Saponins from the leaves of Musanga cecropioides (cecropiaceae) constitute a possible source of potent hypotensive principles. Phytotherapy Research 10(1): 23-27.
- Ayinde BA, Omogbai EKI, Onwukaeme DN (2003) Pharmacognostic characteristics and hypotensive effect of the stem bark of Musanga cecropioides R. Br. (moraceae). West African Journal of Pharmacology and Drug Research 19(1-2): 37-41.
- Ohigashi H, Kaji M, Hoshino J, Jato J, Koshimizu K (1987) The search for useful plants in the tropical rain forest of Cameroon and the biological activities of these plants. Journal of African Studies 30: 1-14.
- Irvine FR (1961) Woody plants of Ghana with special reference to their uses. Oxford University Press, UK, pp. 143-144.
- Bouquet A (1969) Féticheurs et médecines traditionnelles du Congo Brazzaville. Mémoires ORSTROM N°36. Office de la Recherche Scientifique et Technique Outre-Mer. Paris, France, p. 282.
- Adjanohoun EJ, Ahyi AMR, Ake Assi L, Baniakina J, Chibon P, et al. (1988) Contribution aux études ethnobotaniques et floristiques en république populaire du congo. Agence de Coopération Culturelle et Technique, Paris, France, p. 149.
- Burkill HM (1985) The useful plants of West Tropical Africa. Vol. 1(12).
- Lontsi D, Sondengam BL, Ayafor JF, Connolly JD (1987) Cecropiacic acid, a new pentacyclic a-ring seco triterpenoid from Musanga cecropioides. Tetrahedron Letters 28(52): 6683-6686.
- Lontsi D, Sondengam BL, Ayafor JF (1989) Chemical studies on the cecropiaceae-a novel a-ring seco triterpene from Musanga cecropioides. Journal of Natural products 52(1): 52-56.
- Lontsi D, Sondengam BL, Ayafor JF, Tsoupras MG, Taracchi R (1990) Further triterpenoids of Musanga cecropioides-The structure of cecropic acid. Planta Medica 56(3): 287-289.
- Lontsi D, Sondengam BL, Martin MT, Bodo B (1991) Musancropic acids A and B: A-ring contracted triterpenes from Musanga cecropioides. Phytochemistry 30(7): 2361-2364.
- Lontsi D, Sondengam BL, Martin MT, Bodo B (1991) Seco-ring- A triterpenoids from the root wood of Musanga cecropioides. Phytochemistry 30(5): 1621-1624.
- Lontsi D, Sondengam BL, Martin MT, Bodo B. (1992) Musangicic acid, a triterpenoid constituent of Musanga cecropioides. Phytochemistry 31(12): 4285-4288.
- Lontsi D, Sondengam BL, Bodo B, Martin MT (1998) Kalaic acid, a new ursane-type saponin from Musanga cecropioides. Planta Medica 64(2): 189-191.
- Lontsi D, Sondengam BL, Bodo B, Martin MT (1998) Cecropioic acid-a pentacyclic triterpene from Musanga cecropioides. Phytochemistry 48(1): 171-174.
- Lacaille DMA, Frank U, Wagner H (2001) Search for potential angiotensin converting enzymes (ace)-inhibitors from plants. Phytomedicine 8(1): 47-52.
- Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO (2006) Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacrol 105(3): 374-379.
- Dibong SD, Etame LG, Tankeu SE, Okalla EC, Yinyang J et al. (2018) Acute and subacute toxicity study of the combination of aqueous extracts of the trunk bark of Musanga cecropioides r. br. (cecropiaceae) and the fruits of Combretum micranthum g. don (combretaceae). Saudi Journal of Medical and Pharmaceutical Sciences 4(9): 1018-1026.
- Nwidu LL, Oboma YI, Elmorsy E, Carter WG (2018) Hepatoprotective effect of hydromethanolic leaf extract of Musanga cecropioides (urticaceae) on carbon tetrachloride-induced liver injury and oxidative stress. Journal of Taibah University Medical Sciences 13(4): (344-354).
- Adeneye AA (2009) Protective activity of the stem barks aqueous extract of Musanga cecropioides in carbon tetrachloride- and acetaminopheninduced acute hepatotoxicity in rats. Afr J Tradit Complement Altern Med 6(2): 131-138.
- OECD (2001) OECD Guidelines for the testing of chemicals: Test No. 423: Acute Oral Toxicity- Acute Toxic Class Method. Organization for Economic Cooperation and Development, Paris, France.
- Mayer P (1896) Mitt Zool Stn. (12th edn), Naples, Italy, p. 303.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA (2002) Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of swiss albino mice. Scientia Pharmaceutica 70: 135-145.
- Rosidah Y, Sdikun A, Ahmad M, Akowuah GA, Asmawi MZ (2009) Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol 123(2): 244-249.
- Legbosi NL, Ellis TR (2018) Sub-chronic toxicity of hydromethanolic stem bark extract of Musanga cecropioides (urticaceae) in rat. EC Pharmacology and Toxicology 6(3): 76-95.
- Amresh G, Singh PN, Rao CV (2008) Toxicological screening of traditional medicine laghupatha (Cissampelos pareira) in experimental animals. J Ethnopharmacol 116(3): 454-460.
- Mohamed EA, Lim CP, Ebrika OS, Asmawi MZ, Sadikun A, et al. (2011) Toxicity evaluation of a standardised 50% ethanol extract of orthosiphonstamineus. J Ethnopharmacol 133(2): 358-363.
- Dybing E, Doe J, Groten J, Kleiner J, O Brien J et al. (2002) Hazard charecterization of chemicals in food and diet: dose response, mechanism and extrapolation issues. Food Chem Toxicol 40(2-3): 237-282.
- Mukinda JT, Syce JA (2007) Acute and chronic toxicity of aqueous extract of artemisia afra in rodents. J Ethnopharmacol 112(1): 138-144.
- Emerson FS, Shadara AC, Devi PU (1993) Toxic effects of crude extract of plumbago rosea (rokta chitraka) on mice and rats. J Ethnopharmacol 38(1): 79-84.
- Omoruyi SI, Enogieru AB (2018) Musanga cecropioides (cecropiaceae) attenuates carbon tetrachloride-induced non-alcoholic fatty liver disease in wistar rats. Trop J Nat Prod Res 2(11): 482-488.
- Bidhe RM, Ghosh S (2004) Acute and subchronic (28 days) oral toxicity study in rats fed with novel surfactants. AAPS PharmSci 6(2): 1-10.
- Smith GL, Shlipak MG, Havranek EP (2006) Serum urea nitrogen, creatinine, and estimators of renal function. Arch Intern Med 166(10): 1134-1142.
- Fuster V, Eric J, Nabel EG (2005) Pathno-biology of asymptomatic arthrosclerosis leading to symptomatic artherothrombosis. J Am Coll Cardiol 46: 937-941.
- Carmena R, Duriez P, Fruchart JC (2004) Atherogenic lipoprotein particles in atherosclerosis. Circulation 109(23): 1112-1117.
- Kontush A, Chapman MJ (2006) Antiatherogenic small, dense HDLguardian angel of the arterial wall? Nat Clin Pract Cardiovasc Med 3(3): 144-153.
- Yang L, Wen KS, Ruan X, Zhao YX, Wei F, et al. (2018) Response of plant secondary metabolites to environmental factors. Molecules 23(4): 762.
- Tan PV, Mezui C, Orock GE, Njikam N, Dimo T, et al. (2008) Teratogenic effects, acute and subchronic toxicity of the leaf aqueous extract of Ocimum suave Wild (Lamiaceae) in rats. J Ethnopharmacol 115(2): 232- 237.
- Mukinda JT, Eagles PF (2010) Acute and sub-chronic oral toxicity profile of the aqueous extract of Polygala fruticosa in female mice and rats. J Ethnopharmacol 128(1): 236-240.
- Larrey D (2002) Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis 22(2): 145-155.
© 2019 Nyunaï N. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






