- Submissions

Full Text
Advances in Complementary & Alternative medicine
The Effects of Traditional Chinese Medicine on Immunomodulation for Cancer Therapy
Lu Yang1,2,3, Rushdi Fadhil3, BinBin Yang1,3, David Good3,4, Wei Liu1,3, Guoying Ni3,5,6,7, Jasmine Kaur3, Xiao Song Liu5,6,7,Tamim Mosaiab3, Calvin Jessop3, Chengjin Hu2* and Ming Q Wei3*
1 Department of Medical Laboratory, China
2 The 960th Hospital of the PLA Joint Logistics Support Force, China
3 School of Medical Science & Menzies Health Institute Queensland, Australia
4 School of Allied Health, Australia
5 Cancer Research Institute, China
6 Inflammation and Healing Research Cluster, Australia
7 Molecular diagnosis and Target Therapy Laboratory, China
*Corresponding author: Chengjin Hu, The 960th Hospital of the PLA Joint Logistics Support Force, Jinan, 250000, China
Ming Q Wei, Menzies Health Institute Queensland and School of Medical Science, Gold Coast, QLD, 4222, Australia
Submission: February 15, 2019;Published: February 19, 2019

ISSN: 2637-7802 Volume4 Issue2
Abstract
Traditional Chinese medicine (TCM) has been practiced in China and surrounding regions for more than 2000 years. Research has shown that TCM is an effective and alternative option for anticancer therapy. A plethora of Chinese herbal recipes today serve as alternative or complementary medicines to treat cancer and/or alleviate the side effects of conventional therapies. This review focused on the immunoregulatory properties of TCM, introducing their effect on immune cells, immune molecules and immune organs along with discussions of their functions in improvement of cancer immunosuppressive state. Some important examples of these TCM as well as natural components possessing immunomodulatory and/or anticancer actions isolated from traditional Chinese medicines are also discussed.
Keywords: Traditional chinese medicine (TCM); Immunomodulation; Cancer therapy
Abbreviations: DCs: Dendritic Cells; APCs: Antigen Presenting Cells; NK Cells: Natural Killer Cells; MHC: Major Histocompatibility Complex; CTL: Cytotoxic T Cell; Tregs: Regulatory T Cells; TME: Cancer Microenvironment; OSCC: Oral Squamous Cells
Introduction
Cancer is considered to be one of the leading causes of death worldwide [1]. In 2018 alone, there have been an estimated number of 18.1 million new cases diagnosed globally, and approximately 9.6 million cancer deaths [1]. Unfortunately, this number is rising alarmingly every year, especially in some part of the developing world. Although there are various methods of cancer treatments with which some are in the front line of fighting against cancers, they have limitations which have shown ineffectiveness in curbing the progress of cancer. The most conventional and commonly used therapies are surgical, radiotherapy, chemotherapy and some targeted therapies, but each of them has its own limitations with some having side effects [2]. Therefore, it is essential to develop new agents that could induce cancer cell death without damaging healthy cells.
Recently, immunotherapy has become an area of significant progress. Modulation of immune responses can restore and enhance the host’s immune system to control and clear cancers, forming immune memory to provide long-term protection. Traditional Chinese medicine (TCM) has been practiced for more than 2000 years, with immunomodulation and anti-cancer properties, and has the potential to become auxiliary drugs for cancer immunotherapy. TCM are multi-components and multitarget agents which emphasize the overall effect, enhancing the endogenous immunity of the body to cancer in a more holistic way [3]. On the one hand, TCM can boost anticancer immunity by activating, enhancing or restoring the innate and adaptive immunity and coordinating the balance between cytokines. On the other hand, TCM can also reverse the state of immunosuppression, regulate and reshape the inhibitory function of immune cells, and prevent cancer escape. This review discusses the causes of cancer, the characteristics of cancerous cells, the difference between benign and malignant tumours, and the different groups of cancers based on a histological point of view. It explores various cancer treatments such as surgery, chemotherapy, radiotherapy, and immunotherapy, particularly detailing the type of cancers that they can treat, how the treatment work, and potential side effects that they can cause to the patient In this review, we will summarize the various immunomodulatory effects of TCM on cancer.
TCM Enhances Anti-Cancer Activity of Immune Cells
Effect on macrophages
Macrophages are characterized by their plasticity and heterogeneity, which acquire distinct functional phenotypes in respond to environmental cues such as microbial products, damaged cells, activated lymphocytes [4]. Depending on specific signals, macrophages may be polarized into M1 and M2 subtypes. M1 macrophages are proinflammatory and they display microbicidal, anticancer functions via release of soluble enzyme and cytokines. M2 macrophages have immune regulating and suppressive effects, participating in tissue remodeling, parasite suppression and promote cancer cell proliferation to some extent. Studies indicated that cancer-infiltrating macrophages generally tend to M2 phenotype which plays a pro-cancerigenic role [5]. Therefore, targeting macrophages and regulating M1/M2 phenotypes would be an efficient method to promote cancer regression.
Among several researches of TCM effects on macrophages, Liu et al. [6] reported that Polyporus polysaccharide (PPS), the effective ingredients of medicinal fungus Polyporus, has been well documented to have anti-cancer, antioxidant and immunoregulatory abilities. They measured the effect of PPS on macrophage polarization and macrophage activation mechanism in the microenvironment of bladder cancer. The first part of their researches showed that PPS propelled INF-stimulated Raw 264.7 cells toward the M1 phenothpe; enhanced costimulatory molecule expressions of CD40, CD284 and CD86; increased secretion of NO, IL-16 and TNF-α; promoted the phagocytosis and pro-inflammatory capacity. The following studies revealed the mechanism of the stimulating activity of PPS. It turned out that PPS induce IFN-γ- stimulated macrophage activation via activating TLR4-mediated NF-κB signaling pathways. These results indicated that PPS could enhance immunostimulatory activities by regulating macrophage function.
Effect on dendritic cells
Dendritic cells (DCs) are the most potent antigen presenting cells (APCs) whose primary function is to uptake, process and present various antigens, including cancer antigens, to antigenspecific naïve T cells [7]. Meanwhile, they provide a host of other essential signals (costimulatory molecules and cytokines) to drive T cell proliferation and differentiation to occur [8]. Dendritic cells also contribute to the function of other immune cells and help maintain immune memory. They are known as the central link between the innate and adaptive immune response, thus activating DCs could be an effective way to prime T-mediated anticancer immunity.
Researchers found that TCM and their components can activate DCs at the earliest stage of immune response by targeting pattern recognition receptors, like TLR4, Dectin-1, on the surface of DC cells. For instance, Tian et al. [9] reported that Astragalus mongholicus (AMs) has an effect in treating human stomach cancer and the mechanism is regulated by TLR4 mediated NF-κB signal transduction of DCs. Ficus carica L, a traditional plant from China, has traditionally been used in Asian for its anti-cancer properties. Tian et al. [10] found that Ficus carica polysaccharides (FCPS) from Ficus carica L. could effectively activated DCs via dectin-1/Syk pathway as well as promote maturation of DCs, as shown by upregulating costimulatory molecule (CD40, CD80, CD86) and MHCII; and an increased production of cytokines (IL-12, IFN-γ, IL-6, and IL-23).
Not only that, DC can also be stimulated by engulfed antigens. Endocytosis of DC Ganoderma lucidum polysaccharide (GLP) induced phenotypic maturation, including up-regulation of costimulatory factors, increasing phagocytic capacity and releasing NO. Interestingly, endocytic inhibitors sodium azide and brefeldin A were used in cell culture; the maturation phenotypic changes were inhibited. These results suggest that endocytosis also contributes to DC maturation, in addition to stimulating Toll-like receptors (TLRs) [11].
Effect on natural killer cells
Natural killer (NK) cells are innate lymphoid cells crucially important for defense against viruses and for cancer immunesurveillance. After activation, NK cells produce cytotoxic cytokines with direct cytolytic activity or killing infected cells and malignant cells by antibody-dependent cellular cytotoxicity. They also regulate the growth and differentiation of monocytes, dendritic cells and granulocytes by releasing cytokines and chemokines that induce inflammatory responses; and affect subsequent adaptive immune responses [12]. Studies have demonstrated that they make an important impact in the control of cancer progression and metastasis.
Among numerous studies of TCMs on the function of NK cells, Wu et al. [13] found that Lupeol, a triterpene, can induce proliferation of NK cells and inhibit growth of human gastric cancer cell lines BGC823, N87 and HGC27 in an appropriate concentration range (0.1 to 25μg/ml). It was shown that Lupeol increased the ability of NK cells and the expression of PFP, IFN-γ, and CD107a. Further examination suggested that lupinol promoted the proliferation and killing activity of NK cells by activating PI3K/Akt and Wnt/β- Catenin signaling pathways.
Additionally, Lee et al. [14] isolated a glycoprotein with antioxidant and anticancer activity from Zanthoxylum piperitum DC (ZPDC). ZPDC was applied to diethyl nitrosamine (DEN)-treated Balb / c mice with liver cancer in order to observe its effects on liver cancer cells and NK cells. The results suggested that ZPDC (20mg/kg, body weight) can promote the release of perforin and granzyme B, increase the cytotoxic activity of NK cells, promote the expression of apoptosis-related factors, including bid, cytochrome c and caspase-3, in hepatocarcinoma tissues. Collectively, ZPDC glycoprotein enhanced NK cells anti-cancer activity, could be a potential preparation for preventing hepatocarcinogenesis.
Effect on γδT cells
γδT cells are a special subgroup of T-lymphocytes expressing the T cell receptors (TCRs) γ and δ chains, which mainly distributed in the epithelial and mucosal tissues. Although the number of γδ T cells is only 0.5-5% of all T cells, they participate in various immune responses during cancer progression [15]. γδT cells exert anticancer reactions that is characterized by TCR-dependent pathway (antibody-dependent cellular cytotoxicity) or through producing pro-apoptotic molecules perforin, granzyme and inflammatory cytokines IFN-γ and TNF-α. They can induce DCs, NK cells, αβ T cells, and B cells to exert an indirect anticancer effect.
More recently, Sun et al. [16] investigate the functions of Astragalus polysaccharides (APS) on intestinal intraepithelial γδT cells. APS was found to promote proliferation and activity of intestinal intraepithelial γδT cells. APS increase IFN-γ, as well as FasL and GrB mRNA levels from stimulated γδT cells. Moreover, the concentration of TNF-α and IFN-γ was markedly increased while the concentrations of IL-10 and TGF-β were markedly decreased in cancer-bearing mice after treatment with APS. In conclusion, these results indicated that APS could effectively activate intestinal γδT cells, inhibiting cancer growth.
Effect on CD8+T lymphocytes
CD8+ T lymphocytes (also known as Cytotoxic T cell or CTL) are crucial components of anticancer immunity response which kill malignant cells. The activity is dependent on T-cell receptor (TCR) to recognize class I MHC molecules and antigen complexes present on the surface of target cells [17]. CTL directly lysis cancer cells via production of cytotoxic granules containing granzymes and perforin. These cells also indirectly exert anti-cancer effect through secretion of cytokines IFN-γ and TNFs. Clinical studies have found that increased intracanceral CTL infiltration is associated with good clinical outcomes in most cancers, such as melanoma, breast cancer, prostatic and lung cancers [18].
Weikangfu granule compound (WKC) is a clinical prescription drug which comprises polysaccharides, saponin, flavonoids, and essential oil, having therapeutic effects for gastric cancer. Nie et al. [19] evaluated the anticancer and immunomodulatory ability of WKC in cancer-bearing mouse models. In this study, cancer-bearing mice of control groups were treated with cyclophosphamide or isotonic saline but the treatment groups were administered with low and high doses of WKC for consecutive days. On day 11, the following were measured, including weight of mice, cancer sizes, spleens cells and the activity of CTL. The results showed that the cancers were significantly regressed while CTL activities and lymphocyte count were significantly increased in the WKC-treated groups. The study suggested that WKC exhibited anticancer ability as well as immunomodulatory activities, which could improve lymphocyte proliferation and CTL activity for anticancer.
Effect on CD4+ T lymphocytes
It is generally believed that main mechanism of the immune system against cancers has been attributed to CD8+ cytotoxic T cells (CTL)-mediated killing activity. However, studies demonstrated that CD4+ T lymphocytes (Th cells) are essential for the maintenance of effective CD8+ cytotoxicity and the production of memory T cells [20], playing a central role of immune response. Th cells differentiate into two major subtypes known as Th1 and Th2 cells. Th1 are mainly responsible for promoting cellular immune responses, initiating cytotoxic response and displaying anticancer effects, through the production of cytokines IFN-γ, TNF-α, and chemokines. TH2 cells are characterized by the secretion of cytokines IL-4, IL-5 and IL-13 to coordinate humoral immunity and improve allergic inflammatory reactions, playing a suppressive role in cancer immunity [21].
Some studies have found that a common feature in cancer patients is the abnormal proportion of Th1/Th2. In the study of mouse renal cell carcinoma and colon adenocarcinoma models, Ghosh et al. [22] found that with cancer growth and progression, Th1 cell populations gradually decreased, while Th2 cytokine expression increased. There is a lot of evidence that cancer cells themselves could secrete a variety of cytokines that promote their own growth. These cytokines may alter the ratio of Th1 or Th2 by direct action on T cells or subsequent reaction with other immune cells, including dendritic cells (DCs) or macrophages, thus, propelling the transformation of Th1/Th2 balance to Th2 reaction and inhibiting Th1 response [23].
Many studies have suggested that TCM can modulate the balance of Th1 and Th2 ratios, thus regulating T cell-mediated immunity [24]. Wei et al. [25] studied the effect of a Chinese herb component, Astragalus Membranaceus (AG) on Th1/Th2 type cytokine production and gene expression in peripheral blood mononuclear cells (PBMNC) in 37 lung cancer patients and 19 healthy subjects. As for PBMNC in patients group, Th2 exert a dominant position with the level of Th2 transcript factor GATA3 and mRNA of Th2 cytokines IL-4, IL-6 and IL-10 were higher than Th1 transcript factor T-bet and mRNA of Th1 cytokines IFN-γ, and IL-2. When adding AG to PBMNC culture, the predominant trend of Th2 was suppressed, and the balance between Th1/Th2 lean toward Th1. These evidences showed that AG could reverse the dominant position of Th2 in lung cancer patients, which could be used as a potential and valuable alternative in cancer treatment.
Effect on B lymphocytes
The basic function of B lymphocytes is to differentiate into plasma cells by antigen stimulation, followed by generating and secreting specific antibodies to regulate humoral immunity. Studies have found that cancer infiltrating B cells can produce cancerreactive antibodies which promote cancer killing of NK cells, phagocytosis of macrophages, and initiation of CD4+ and CD8+T cells to inhibit cancer growth [26].
More than 10 years ago, Zhang et al. [27] reported that GLIS, a proteoglycan isolated from the fruiting body of a Chinese herb, the fungus Ganoderma Lucidu, is a B-cell stimulating factor. GLIS actively stimulated B cells proliferation and activation and promoted the secretion of immunoglobulin from B cells. The percentage of B cells in mouse spleen was increased three to four-fold after GLIS stimulation. GLIS also led to B cells enlarged, expressed CD71 and CD25 on the cell surface. Subsequently, this group investigated the functional mechanisms of GLIS. Unlike other immune-stimulating reagent, GLIS induced B cell proliferation but had no effect on T cells. The results indicate that the activation of B cells is dependent on the increase in the expression of PKCα and PKCγ in B cells.
Another polysaccharide ASP is extracted from traditional herbal TCM Acanthopanax koreanum (AK), which has a typical specificity in activating B cells and macrophages, but not T cells. They found that ASP could significantly stimulated the proliferation of B cells and as well as the activation of macrophages, increasing of antibodies and the production of cytokine (TNF-α, IL-1β and IL-6) in B cells and macrophages, respectively. Further studies found that the treatment of the cells with TLR4 and TLR2 antibodies markedly reduced the activities of ASP of B cells and macrophages prior to ASP. Moreover, ASP was capable of activating the TLR signaling cascades including Erk1/2, p38 and JNK, and the transcription factor TNF-β via binding with TLRs. In general, these results indicated AK polysaccharide interacts with B cell and macrophage surface receptors such as TLR4 and TLR2 to activate immune system [28].
TCM Regulates the Anti-Cancer Ability of Immune Molecules
Effect on cytokines
Cytokines serve predominantly as molecular messengers that involved in intercellular communication, inflammatory response amplification and immunomodulation [29]. Although signals can be transmitted by direct interaction between cells in the immune system, secretion of cytokines can propagate immune signals in a more efficient and faster manner [30]. Cytokines characterized by inducing activated immune cells and stromal cells in the immune microenvironment and enhance cytotoxic effector cells cancericidal efficacy, contributes to eliminate cancer. However, cancer cells themselves can produce a variety of cytokines which may act as autocrine growth factors (e.g., IL-8) or immunomodulators (e.g., IL-10, TGF-β) to inhibit anti-cancer immunity and promote cancer progression [31].
A TCM formula named Fuzheng Guben has been used in China for a long time due to its well-known preventive and treatment ability for various cancers. Fuzheng Yiliu Decoction (FYD), a typical example of Fuzheng Guben recipe, is a combination of Astragalus membranaceus, Ligustrum lucidum, Ganoderma lucidum and Rhizoma dioscorea. Chen et al. [32] reported that FYD containing serum markedly inhibited the growth and proliferation of hepatoma cells by increasing the levels of IL-2 and TNF-α in vivo in animal models of cancers.
Effect on complement system
The complement system plays a supporting role in antibodymediated cancericidal responses. Currently recognized mechanisms for complement clearance of cancer cells are mainly in the following two aspects.
A. With the help of CD4+ T cells and APCs, B cells respond to soluble antigens or membrane-expressing antigens secreted by cancer cells and produce anti-cancer antibodies. This antibody activates the complement system and kills cancer cells by antibody dependent cell mediated cytotoxicity (ADCC), inhibiting cancer growth.
B. Complements can also bind to the cell membrane surface antigen to become a complex to activate the classical pathway of complement system, forming membrane attack complex (MAC) to form a hydrophilic transmembrane pore cells on the cell membrane to lyse the cancer cells, which is complement-dependent cytotoxicity (CDC).
Some researchers have reported that ingredients from Chinese medicine can activate the complement system. Polysaccharide (RR1) isolated from TCM Tinospora cordifolia activated the alternate pathway (C3a) of complement activation and there was very little effect on the classical pathway (C4a) [32]. While some ingredients mainly activate complement system through classical pathway, such as Bupleurum polysaccharide [33] and liquor-rice polysaccharide [34], Acidic polysaccharide from TCM Lithospermum euchromum Royle activates complement via both the alternative and classical pathways [35].
TCM Improves the State of Immune Organs
The immune organs are places where immune cells develop, settle and exert effects, which is the basis of controlling host immunity and cancer defense. Immune organs are composed of central immune organs (bone marrow, thymus) and peripheral immune organs (spleen, lymphoid tissue, mucosa related tissue, etc.). The human body produces hundreds of millions of immune cells, including various lymphocytes and various phagocytic cells, which are derived from human immune organs. At the same time, these immune cells can monitor cancerigenesis and release anticancer cytokines to exert anti-cancer efficacy, becoming the main force against cancers. Therefore, the status of immune organs is essential in terms of suppressing proliferation, invasion, and dissemination of the cancer [36].
Studies have found that various Chinese medicines have a significant role in promoting immune organ’s growth and immune function within. More recently, Wang et al. [37] demonstrated that PGFP1, one polysaccharide isolated from Panax ginseng C. A. Mey, has an anticancer effect, as well as the ability to increase the weight of immune organ and splenocyte proliferation in cancer-bearing mice. In the positive control group of these mice, administration of cyclophosphamide (CTX, a kind of chemotherapeutic drug) significantly reduced spleen and thymus weight and suppressed ConA- or LPS-induced T cells proliferation. However, these decreases were greatly reversed by treatment with GFP1. At the same time, the author found that PGFP1 can effectively inhibit cancer growth and metastasis, activate NK cells, and promote the production of IL-2 and IFN-γ along with the increase of the CD4+/ CD8+ ratio of cancer-bearing mice. It is concluded that GFP1 may be effective in inhibiting cancer growth and metastasis via improving immune responses.
TCM Reverses Cancer Immunosuppression
Current research of TCM mainly focused on the unilateral immunity promotion. A large number studies have found that the existence of immunosuppressive components is the key to cause failure of a given cancer immunotherapy. Immunosuppressive ingredients mainly include myeloid derived suppressor cells (MDSCs), regulatory T cells and cancer associated macrophages (TAMs) as well as immunosuppressive factors IL-10, IL-16 and PD-1. These immunosuppressive cells and factors shape the microenvironment that is conducive to cancer growth, ultimately causing cancer escape.
Effect on regulatory T cells
Regulatory T cells (Tregs) is a subpopulation of T cell with immunosuppression capacity that involve in regulating immune responses as well as maintaining immune homeostasis and selftolerance. While Tregs are reprogrammed by cancer cells in the cancer microenvironment (TME), they enhance suppressor functions and served predominantly as an accomplice for cancer escape [38]. Tregs also hinder CD8+ T cell anticancer immunity responses and suppress NK cells and DCs via secretion of immunosuppressive cytokines and intercellular contact. Numerous studies have shown that the number of Treg cells is frequently increased in cancer-bearing mice, and inhibition of Treg can inhibit cancer growth and enhance anti-cancer immunity [39].
It has been reported that Radix Glycyrrhizae polysaccharide (GP), a major active ingredient isolated from TCM Radix Glycyrrhizae, exhibited ability to regulate Treg cells and Th1/ Th2 cytokines in vivo. He et al. [40] found that treatment with GP inhibited cancer progression in H22 hepatocarcinoma cancerbearing mice; reduced the proportion of Treg cells in peripheral lymphoid organs, and significantly decreased IL-10 and TGF-β production in serum. Meanwhile, GP balanced Th1/Th2 cytokines, enhancing the production of IL-2 and IL-12 p70. Research into molecular mechanism revealed that GP-mediated decreases in the proportion of Treg cells in cancer-bearing mice through the reductions in Foxp3 expression.
Effect on myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells (IMC) which comprise precursors for granulocytes, DCs or macrophages that are involved in the occurrence and development of cancers [41]. They are characterized by the production of nitric oxide (NO), reactive oxygen species (ROS) and cytokines (IL)-10 and transforming growth factor (TGF)-β1, as well as the secretion of angiogenic factors that promote cancer angiogenesis, showing remarkable immunosuppressive and cancerigenic activities [42]. Hence, MDSCs can be considered as the major driver in cancer-mediated immunosuppression.
Recent study in colorectal cancer xenograft bearing mice model showed that ginseng-derived compound K (C-K) had significant effect on MDSCs. It was found that the percentages of apoptosis in early and late MDSCs was higher in the C-K treatment group than in the control group. C-K also significant decreased the expressions of immunosuppression-related genes Cox-2 and Arg-1, and inhibited the secretion of IL-1β, IL-6, and IL-17. The activity of promoting CT26 cancer progression in mice was significantly attenuated [43].
Effect on programmed death- 1(PD-1)
Cancer cells can exploit a variety of pathways to evade immune surveillance and endogenous “immune checkpoints” [44]. Programmed death- 1 (PD-1) is an important immune-checkpoint receptor mainly expresses on immune effector cells and stromal cells [45], which interacts with its two ligands, PD-L1 or PD-L2 to transmit negative signals [46]. When PD-1 encounters the immunosuppressive ligand PD-L1 expressed by cancer cells, the binding of PD-1 and PD-L1 can inhibit T-cell anticancer activity and thus be conducive to immune escape of cancers. Therefore, targeting PD-1/PD-L1 signaling pathway is a new immunotherapy for human cancers.
A TCM Zhihuang Fuzheng Soft Capsule (ZFSC), which is composed of Ganoderma spore oil, Ganoderma extract, Phellinus igniarius extract, and Panax notoginseng saponins, showed an obvious effect in inhibiting cancer growth and enhancing immune response. Bao et al. [47] studied the anticancer effect of ZFSC on HT-1080, A-549, and HCT-8 cancer-bearing mice. They found that ZFSC inhibited the growth of human fibrosarcoma and human lung adenocarcinoma cells in transplant mice model but had no obvious effect on human colon cancer. Further studies demonstrated the effect was mediated by reducing IFN-γ and TNF-α in the serum and inhibiting PD-1 and PDL1. These results suggested that ZFSC hindered the PDL1/PD-1 pathway, which may resulted in the inhibition of cancer growth (Table 1).
Anticancer Effect of Active Ingredients of TCM
The therapeutic effect of Chinese herbal compounds is the result of coordination from numerous natural compounds [48]. With the development of phytochemistry and separation technology, many TCM natural compounds and active ingredients have been carefully studied pre-clinically and in clinical trials, which have shown tremendous potential in the development of novel anti-cancer natural products (Table 1).
Polysaccharides
Polysaccharides are the main immunologically active substances in many traditional Chinese medicines, widely existing in plants and microorganisms. They have significant anticancer and immune-enhancing activity and less side effects on the human body. Therefore, research on the effects of Chinese medicine polysaccharides on cancer and immune function has become the focus of traditional Chinese medicine research.
Table 1:Different groups of active compounds isolated from plants/TCM.
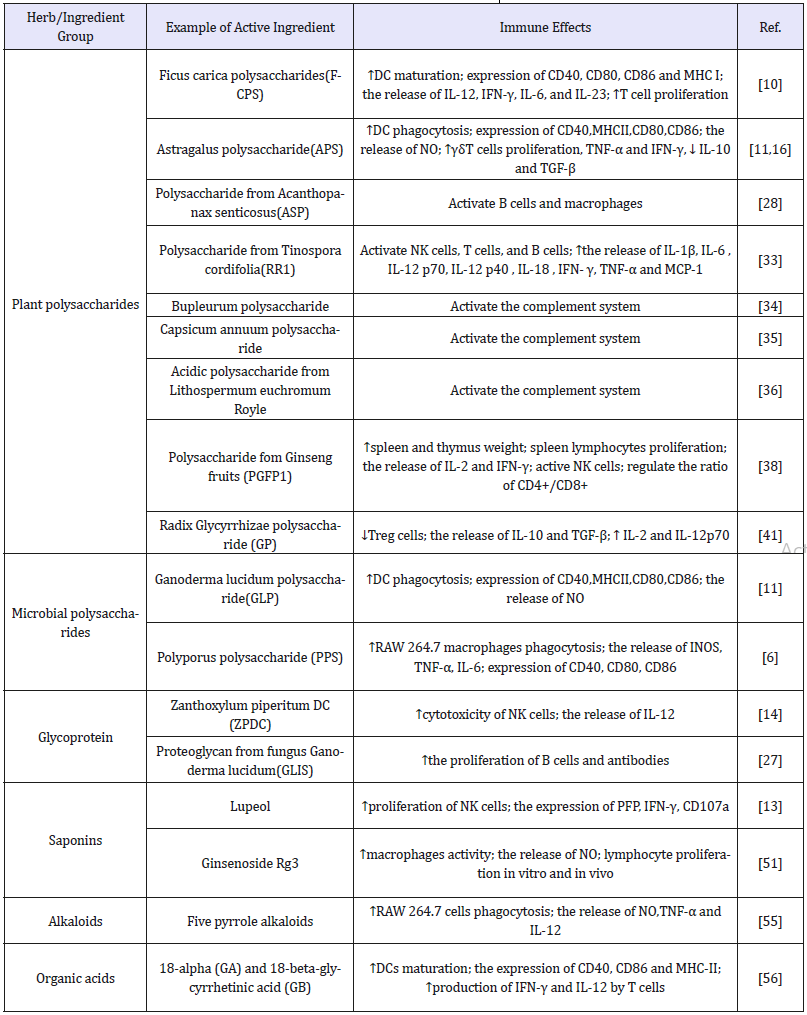
Plant polysaccharides: The sugar in plants accounts for 80% of the dry weight of plants, and the variety of plant polysaccharides is easy to industrialize. Studies have shown that plant polysaccharides can regulate the immune function of the body by binding to various receptors on the surface of immune cells and activating different signaling pathways. For instance, the anticancer effect of Campanulaceae polysaccharides is mainly manifested by binding to TLR-4 to mediate macrophage activation, promoting TNF-α and NO generation [37].
Microbial polysaccharides: Microbial polysaccharides are biopolymers produced by microorganisms such as bacteria, fungi, and cyanobacteria, which have a protective effect on microorganisms. The microbial polysaccharides used in clinical practice are mainly fungal polysaccharides, and most of them have biological activities such as anti-cancer, immune regulation, anti-aging, anti-infection. The study found that the combination of lentinan and S-1 (oral anticancer agent) could down-regulate the level of thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD) and orotate phosphoribosyltransferase (OPRT) in serum. It is safe and shows therapeutic effects on advanced oral squamous cells (OSCC) [49].
Saponins
Saponins are a complex class of glycosides composed of saponins and sugars and uronic acids, which are widely presented in Ginseng, Panax, Licorice, Bupleurum, Tragacanth, Anemarrhena and other Chinese herbal medicines. The molecular mechanisms of cancer suppression employed by saponins mainly involve the improvement of immunity, the inducement apoptosis of cancer cells, the reducement of invasion, metastasis and angiogenesis of cancer.
A previous study showed that a ginsenoside Rg3-fortified red ginseng preparation (Rg3-RGP) significantly exerts anticancer activities via immunopotentiation, without causing side effects as seen in doxorubicin [50]. In addition, a new drug of traditional Chinese medicine Shenyi capsule, ginsenoside Rg3 monomer preparation, has been used in clinical to treat various types of cancer, such as lung cancer, breast cancer and gastrointestinal cancers in China [51]. The combination of Shenyi capsule with chemotherapy could improve the life expectancy of cancer patients through promoting the host immunity and inhibiting cancer angiogenesis [52].
Alkaloids
Alkaloids are a class of naturally occurring compounds that contain ring structure and primarily basic nitrogen atoms [53]. Accumulated evidence demonstrates that alkaloids exhibit a wide range of bioactivities including anti-inflammatory, antiviral, antimicrobial and anti-cancer, showing their great potential for application.
There are studies which indicated alkaloids can inhibit the expression of COX-2 in vivo and in vitro to exert anticancer activity [53]. Moreover, Pyrrole alkaloids extracted from the fruits of Morus Alba has significantly activated macrophage activity, which can increase the phagocytic capacity of macrophages and promote the production of nitric oxide, TNF-α and IL-12 [54].
Organic acids
The organic acids in traditional Chinese medicines rarely exist in free form, and most of them exist in the form of salts with metal ions or alkaloids such as potassium, sodium and calcium. Extensive research found organic acids show diverse pharmacological activities. For example, oleic acid has anti-cancer effect, protocatechuic acid has antibacterial effect, and anthranilic acid in Folium Isatidis has anti-endotoxin, anti-inflammatory and anti-oxidation functions. Bordbar et al. [55] observed that 18-α (GA) and 18-β -glycyrrhetinic acid (GB) could insert their immunomodulatory effects by promoting DC maturation and regulating Th1/Th2 balance.
Conclusion
TCM and its active ingredients showed various abilities in regulating immune cells, cytokines, immune organs, and improving immune suppression status to inhibit cancerigenesis, growth and metastasis (Table 1). Significant progresses have now been made in the understanding of how TCM works in terms of regulating immune responses, but the use of Chinese herbs for effective management of cancer is still a long way from worldwide clinical application. At present, even though the use of TCM is increasing fast, the breadth and depth of the molecular mechanism of the TCM action is yet to be fully elucidated. Also, there is a need to combine TCM with Western medicine, and the use of molecular biology, modern immunology and pharmacology to dither the mechanism of action and pharmacokinetics of TCM, and its active ingredients, to find their active ingredients and more molecular targets of action. TCM is believed to have the characteristics of low drug resistance, low side effects and few adverse reactions, however, there is a void in the study of the cross-reaction and auxiliary effects between TCM and other therapies as well as whether TCM can be used in the prevention of cancer [56].
Acknowledgement
Thanks goes to Weifang Medical University for the collaboration program which enabled BBY, LY and WL to visit Griffith University.
References
- Christensen J (2018) Cancer will kill nearly 10 million people this year, report estimates. Cable News Network.
- Dai SX, Li WX, Han FF, Guo YC, Zheng JJ, et al. (2016) In silico identification of anti-cancer compounds and plants from traditional Chinese medicine database. Sci Rep 6: 25462.
- Nie J, Zhao C, Deng LI, Chen J, Yu B, et al. (2016) Efficacy of traditional Chinese medicine in treating cancer. Biomed Rep 4(1): 3-14.
- Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3): 787-795
- Genard G, Lucas S, Michiels C (2017) Reprogramming of cancerassociated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol 8: 828.
- Liu CP, Zhang X, Tan QL, Xu WX, Zhou CY, et al. (2017) NF-κB pathways are involved in M1 polarization of RAW 264.7 macrophage by polyporus polysaccharide in the cancer microenvironment. PLoS One 12(11): e0188317.
- Ma Y, Shurin GV, Peiyuan Z, Shurin MR (2013) Dendritic cells in the cancer microenvironment. J Cancer 4(1): 36-44.
- Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL (2015) Cancerinfiltrating dendritic cells in cancer pathogenesis. J Immunol 194(7): 2985-2991.
- Tian Y, Li X, Li H, Lu Q, Sun G, et al. (2014) Astragalus mongholicus regulate the Toll-like-receptor 4 meditated signal transduction of dendritic cells to restrain stomach cancer cells. Afr J Tradit Complement Altern Med 11(3): 92-96.
- Tian J, Zhang Y, Yang X, Rui K, Tang X et al. (2014) Ficus carica polysaccharides promote the maturation and function of dendritic cells. Int J Mol Sci. 15(7): 12469-12479.
- Zhu N, Lv X, Wang Y, Li J, Liu Y, et al. (2016) Comparison of immunoregulatory effects of polysaccharides from three natural herbs and cellular uptake in dendritic cells. Int J Biol Macromol 93(Pt A): 940- 951.
- Wu J, Lanier LL (2003) Natural killer cells and cancer. Adv Cancer Res 90: 127-156.
- Wu XT, Liu JQ, Lu XT, Chen FX, Zhou ZH, et al. (2013) The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharmacol 16(2): 332-340.
- Lee J, Lee SJ (2014) Lim KT ZPDC glycoprotein (24kDa) induces apoptosis and enhances activity of NK cells in N-nitrosodiethylamineinjected Balb/c. Cell Immunol 289(1-2):1-6.
- Zhao Y, Niu C, Cui J (2018) Gamma-delta (γδ) T cells: friend or foe in cancer development? J Transl Med 16(1): 3.
- Sun S, Zheng K, Zhao H, Lu C, Liu B, et al. (2014) Regulatory effect of astragalus polysaccharides on intestinal intraepithelial γδT cells of cancer bearing mice. Molecules 19(9): 15224-15236.
- Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F (2018) Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol 9: 14.
- Feldmeyer L, Gaide O, Speiser DE (2013) Clinical implications of CD8+ T-cell infiltration in frequent and rare cancers. J Invest Dermatol 133(8): 1929-1932.
- Nie X, Shi B, Ding Y, Tao W (2006) Anticancer and immunomodulatory effects of weikangfu granule compound in cancer-bearing mice. Curr Ther Res Clin Exp 67(2): 138-150.
- Yo-Ping L, Chung JJ, Shu-Ching C (2011) The roles of CD4+ T cells in cancer immunity. ISRN Immunology 2011: 1-6.
- Kim HJ, Cantor H (2014) CD4 T-cell subsets and cancer immunity: the helpful and the not-so-helpful. Cancer Immunol Res 2(2): 91-98.
- Ghosh P, Komschlies KL, Cippitelli M, Longo DL, Subleski J, et al. (1995) Gradual loss of T-helper 1 populations in spleen of mice during progressive cancer growth. J Natl Cancer Inst 87(19): 1478-1483.
- Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze (1999) MT Th1/ Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol 21(3): 339-359.
- Jiang MH, Zhu L, Jiang JG (2010) Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin Ther Targets 14(12): 1367-1402.
- Wei H, Sun R, Xiao W, Feng J, Zhen C, et al. (2003) Traditional Chinese medicine Astragalus reverses predominance of Th2 cytokines and their up-stream transcript factors in lung cancer patients. Oncol Rep 10(5): 1507-1512.
- Yuen GJ, Demissie E, Pillai S (2016) B lymphocytes and cancer: a lovehate relationship. Trends Cancer 2(12): 747-757.
- Zhang J, Tang Q, Zimmerman KM, Reutter W, Fan H (2002) Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum. Life Sci 71(6): 623-638.
- Han SB, Yoon YD, Ahn HJ, Lee HS, Lee CW, et al. (2003) Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int Immunopharmacol 3(9): 1301-1312.
- Peters M (1996) Actions of cytokines on the immune response and viral interactions: An overview. Hepatology 23(4): 909-916.
- Cytokines in cancer immunotherapy. Conference Proceeding.
- Cytokines and immune response in the cancer microenvironment. Conference proceeding.
- Chen XZ, Cao ZY, Liao LM, Liu ZZ, Du J (2014) Application of serum pharmacology in evaluating the anticancer effect of Fuzheng Yiliu Decoction from Chinese medicine. Chin J Integr Med 20(6): 450-455.
- Nair PK, Rodriguez S, Ramachandran R, Alamo A, Melnick SJ, et al. (2004) Immune stimulating properties of a novel polysaccharide from the medicinal plant Tinospora cordifolia. Int Immunopharmacol 4(13): 1645-1659.
- Pectic polysaccharides from Chinese herbs: structure and biological activity.
- Zhao JF, Kiyohara H, Yamada H, Takemoto N, Kawamura H (1991) Heterogeneity and characterization of mitogenic and anticomplementary pectic polysaccharides from the roots of Glycyrrhiza uralensis Fisch et DC. Carbohydr Res 219: 149-172.
- Yamada H, Cyong JC, Otsuka Y (1986) Purification and characterization of complement activating-acidic polysaccharide from the root of Lithospermum euchromum Royle. Int J Immunopharmacol 8(1): 71-82.
- Wang Y, Huang M, Sun R, Pan L (2015) Extraction, characterization of a Ginseng fruits polysaccharide and its immune modulating activities in rats with Lewis lung carcinoma. Carbohydr Polym 127: 215-221.
- Whiteside TL (2018) FOXP3+ Treg as a therapeutic target for promoting anti-cancer immunity. Expert Opin Ther Targets 22(4): 353-363.
- The role of regulatory T cells in cancer. Conference proceeding.
- He X, Li X, Liu B, Xu L, Zhao H, et al. (2011) Down-regulation of Treg cells and up-regulation of TH1/TH2 cytokine ratio were induced by polysaccharide from Radix Glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules 16(10): 8343-8352.
- Fleming V, Hu X, Weber R, Nagibin V, Groth C (2018) Targeting myeloidderived suppressor cells to bypass cancer-induced immunosuppression. Front Immunol 9: 398.
- Umansky V, Blattner C, Gebhardt C, Utikal J (2016) The role of myeloidderived suppressor cells (MDSC) in cancer progression. Vaccines (Basel) 4(4).
- Wang R, Li Y, Wang W, Zhou M, Cao Z (2015) Compound K suppresses myeloid-derived suppressor cells in a mouse model bearing CT26 colorectal cancer xenograft. Nan Fang Yi Ke Da Xue Xue Bao 35(5): 748- 752.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26): 2443-2454.
- Shi L, Chen S, Yang L, Li Y (2013) The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 6(1): 74.
- Jin HT, Ahmed R, Okazaki T (2011) Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol 350: 17-37.
- Bao Y, Pan X, Jin Y, Gao Y, Cui X (2016) Antitumour effect of zhihuang fuzheng soft capsules on cancer-bearing mice. Evid Based Complement Alternat Med 2016: 7503726.
- Zhou J, Zhou T, Jiang M, Wang X, Liu Q, et al. (2014) Research progress on synergistic anti-cancer mechanisms of compounds in traditional Chinese medicine. J Tradit Chin Med 34(1): 100-105.
- Harada K, Itashiki Y, Takenawa T, Ueyama Y (2010) Effects of lentinan alone and in combination with fluoropyrimidine anticancer agent on growth of human oral squamous cell carcinoma in vitro and in vivo. Int J Oncol 37(3): 623-631.
- Park D, Bae DK, Jeon JH, Lee J, Oh N, et al. (2011) Immunopotentiation and anticancer effects of a ginsenoside Rg3 -fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol 31(3): 397-405.
- Sun M, Ye Y, Xiao L, Duan X, Zhang Y, et al. (2017) Anticancer effects of ginsenoside Rg3 (Review). Int J Mol Med 39(3): 507-518.
- Lu P, Su W, Miao ZH, Niu HR, Liu J, et al. (2008) Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin J Integr Med 14(1): 33-36.
- Hashmi MA, Khan A, Farooq U, Khan S (2018) Alkaloids as Cyclooxygenase Inhibitors in Anticancer Drug Discovery. Curr Protein Pept Sci 19(3): 292-301
- Kim SB1, Chang BY, Jo YH, Lee SH, Han SB, et al. (2013) Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J Ethnopharmacol 145(1): 393-396.
- Bordbar N, Karimi MH, Amirghofran Z (2014) Phenotypic and functional maturation of murine dendritic cells induced by 18 alpha- and betaglycyrrhetinic acid. Immunopharmacol Immunotoxicol 36(1): 52-60.
- Jiao L, Bi L, Lu Y, Wang Q, Gong Y, et al. (2018) Cancer chemoprevention and therapy using chinese herbal medicine. Biol Proced Online 20: 1.
© 2018 Ming Q Wei. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






