- Submissions

Full Text
Advances in Complementary & Alternative medicine
Managing the F:B Ratio in DM; A Review of the Role of Firmicutes and Bacteroidetes in Diabetes Mellitus
Faiza Ahmed1, Nicholas A Kerna1,2* and Orien L Tulp1
1 College of Medicine, University of Science, Arts and Technology, Montserrat, BWI
2 Suriwongse Medical Center, Thailand
*Corresponding author: Nicholas A Kerna, College of Medicine, University of Science, Arts and Technology, 4288 Youngfield Street, Wheat Ridge, CO 80033, USA, E-mail: Nicholas.Kerna@usat.edu
Submission: January 29, 2019;Published: February 04, 2019

ISSN: 2637-7802 Volume4 Issue1
Abstract
Globally, the incidence of diabetes continues to rise and significant resources are spent on its prevention and treatment. However, the pathogenesis of this disease has yet to be fully understood or explained. Current research indicates that diabetes, especially type 2 diabetes, is highly correlated to an abnormal intestinal microbiota. Bacteria in the gut are essential for numerous metabolic processes including the production of hormones and neurotransmitters and the biosynthesis of vitamins and amino acids. Several studies have shown that gut bacteria play a significant role in controlling type 2 diabetes. The following research aims to show how gut bacteria can be manipulated to improve outcomes in managing diabetes mellitus. This review of research was conducted to determine the role of the gut bacteria in modulating metabolic health and insulin resistance. Various articles, journals, and books were reviewed, and a meta-analysis of the results conducted. From this review of literature, it was noted that alteration of gut bacteria to a more natural balance might reduce oxidative stress on the pancreas and enhance cell permeability glucose uptake. However, an inimical alteration in gut bacteria can spur the overgrowth of certain bacterial species that contribute to diabetes. Thus, it is advised to consume requisite dietary fibers to maintain or restore the gut microbiome in promoting healthy body weight and blood glucose level.
Preface
Diabetes mellitus (DM) affects approximately 150 million people worldwide according to the data from the World Health Organization (WHO) [1]. The number of people affected by DM is likely to double by 2025 due to population growth, an unhealthy diet, sedentary lifestyle, obesity, and aging. Currently, DM is primarily managed with pharmacological agents (such as insulin) or insulin secretagogues (such as metformin). Nonpharmacologic management is also used, eg., dietary regulation, exercise, and psychological counseling. It has been shown that only 40% of DM patients respond satisfactorily to glucose control using secretagogues or insulin injections [2]. According to the World Health Organization (WHO), countries allocate vast sums of money and dedicate massive amounts of resources to help manage DM resulting in an overwhelming burden to families and state governments [1]. As such, there is a desperate need to explore and develop less expensive alternative treatment options with fewer side effects (as compared to conventional and readily available medicines) particularly for countries with limited resources.
Keywords: Alternative treatment; Bacteroidetes; Diabetes mellitus; Firmicutes; Gut bacteria; Insulin; Microbiome; Oxidative stress; Pancreas
Abbreviations: DM: Diabetes Mellitus; IFN-γ: Interferon-Gamma; IL-1: Interleukin-1; IL-2: Interleukin-2; T2DM: Type 2 Diabetes Mellitus; WHO: World Health Organization
Introduction
Diabetes mellitus (DM) is a chronic disease condition that is characterized by the inability of the body to properly regulate blood sugar leading to fluctuations between hyperglycemia and hypoglycemia with no apparent point of balance [3]. The inability to regulate blood glucose level results from insufficient insulin production by the pancreas. DM is classified into type 1 (which results from the inability of the pancreas to produce insulin) and type 2 (which is a relative insulin deficiency) [4].
DM was initially a lifestyle health risk mainly associated with an affluent lifestyle in developed countries. However, current statistics indicate, diabetes is afflicting individuals regardless of social status or economic class. It is no longer the “executive disease” it once was [5]. According to Gruber & Haller (2014), diabetes mellitus is a genetic disease and its expression is controlled by such factors as diet, physical activities, and medication. Current studies have shown the occurrence of DM can be influenced by certain microbes that invade or inhabit the intestines. Thus, there is a call to probe the diabetes-microbe connection. Understanding and manipulating this connection might be utilized in the management of DM [6].
Discussion
A survey by Gardner et al. (2012) established that human resident microbiota play vital roles in health maintenance; thus, the need to explore its role in managing metabolic syndromes, such as DM. According to Meo (2012), [disadvantageous] alteration in the [normal and healthy] microbiota influences the development of type 2 diabetes and the comorbidities thereof, e.g., retinopathy, diabetic foot ulcers, and nephropathy. Conversely, the favorable manipulation of the microbiome can aid in the management and treatment of DM and its sequelae (Figure 1) [7].
Figure 1:The diet-gut microbiota connection in metabolic disease
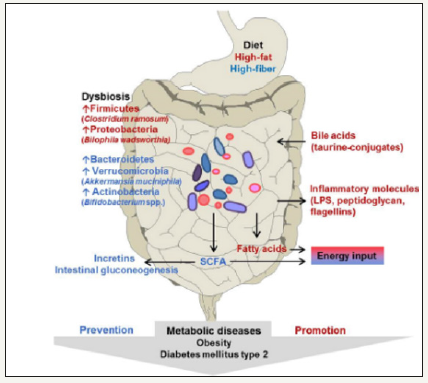
Note: Reproduced from Woting and Blaut (2016). The Intestinal Microbiota in Metabolic Disease [9].
The composition of gut microbiota varies widely between healthy and sick individuals depending on the disease condition. Again, it is for this reason that the manipulation of intestinal microflora can be utilized in managing various diseases, namely DM [8]. Although the cause-effect relationship between the alterations in gut microbiota and disease is not always clear, targeting gut microflora might offer possibilities for managing certain disease conditions. There is a growing understanding of how the microflora is linked to obesity-related diabetes mellitus providing a new potential pathway for managing DM [9].
The gut microbiota participates in energy harvesting; hence, an aberration or alteration in the microbiota may account, at least in part, for the development of obesity. DM is one of the disorders associated with abnormal energy distribution and low-level chronic inflammation among obese individuals. According to Niafar (2012), gut bacteria play an essential role in the progression of prediabetic conditions, such as insulin resistance. Obese individuals show an altered composition of gut microbiota particularly elevation of the Firmicutes: Bacteroidetes (F:B) ratio [8]. The transplantation of gut bacteria from obese individuals to animals negatively affected energy harvesting processes in those animals. This finding from the transplantation of gut bacteria from obese humans to animals strongly suggests there may be a method the gut bacteria can be manipulated to control DM [10].
Shalem & Weiss (2015) illustrated that adverse alteration of microbiota in obese individuals affects intestinal permeability. This alteration increases metabolic endotoxin secretion resulting in chronic low-level inflammation and the pathogenesis of insulin resistance [11]. Commensal bacterial species (Akkermamsia muciniphilis, Escherichia coli, and Bacteroides thetaiotaomicron) have shown some influence on intestinal mucus and the glycocalyx layer altering intestinal permeability. This influence on permeability may also apply to glucose molecules [1].
Nabavi et al. (2015) showed the deleterious cycle between altered microbiota and low-level inflammation observed in obese patients is causative in developing type 2 diabetes mellitus (T2DM). T2DM patients exhibit altered intestinal microflora based on an altered F:B ratio [12]. Decreasing the level of Firmicutes in the gut will reduce insulin resistance and other factors that lead to the development of T2DM [2]. Firmicutes bacteria alter host energy metabolism by usurping polysaccharide loci mechanisms leading to increased levels of bacteria inflammatory molecules in the intestines, such as LPS, flagellin, and peptidoglycans. This increase accelerates the inflammatory process in those suffering T2DM [2]. Gastric bypass surgery has been utilized in certain diabetic patients resulting in a beneficial alteration of the gut microbiome and normalizing blood glucose levels especially in T2DM patients [13].
Figure 2:Probiotics can enhance immunity, increase β-cell proliferation, and decrease β-cell apoptosis.
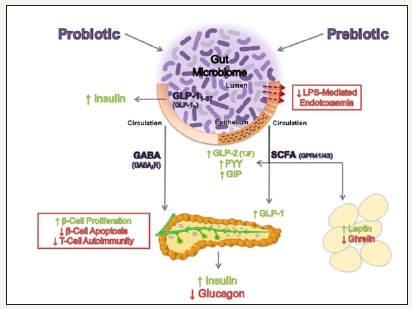
Note: Reproduced from Patterson, Ryan, and Cryan (2016). Gut microbiota, obesity, and diabetes [16].
The gut microbiota can be manipulated by using probiotic strains; certain strains can influence blood glucose homeostasis (Figure 2). Probiotics act as immune system modulators against (altered) microbiota-induced chronic inflammation resulting in obesity-induced DM [14]. Also, some probiotics prevent the onset of DM by down-regulating inflammatory interferon-gamma (IFN-γ) and interleukin-1 (IL-1) or -2 (IL-2). In some cases, probiotics may stimulate a rise in interleukin-10 (IL-10) levels; low IL-10 levels are associated with metabolic syndrome and T2DM [5].
Scott, Antoine & Midtvedt (2015) reported Lactobacillus reuteri GMNL-263 suppressed serum glucose, leptin, GLP-1 level, TNFalpha, insulin, glycated hemoglobin, interleukin IL-6, C-protein, GLUT-4 gene expression, and PPAR-gamma [15,16]. Insulin, GLUT- 4, and PPAR-gamma demonstrated considerable influence on the development and progression of DM. Thus, regulating their production and actions by ingesting certain strains of Lactobacillus could contribute to the management of DM. Lactobacillus strains aid in lowering the ratio of Firmicutes to Bacteroidetes in the gut. This alteration in the F:B ratio inhibits the development and progression of DM. Certain probiotic strains demonstrate favorable antioxidant effects against chronic inflammation. This antioxidant characteristic makes it plausible and possible to alleviate pancreatic oxidative stress that leads to apoptosis of insulin-secreting pancreatic beta cells [16-18].
Conclusion
DM is a metabolic disease that results from the inability of the body to control and maintain proper blood glucose level. Manipulation of gut microflora may be a fundamental factor in reducing oxidative stress on the pancreas and enhancing cell permeability for glucose uptake. Lowering the ratio of Firmicutes to Bacteroidetes is beneficial in managing obesity and obesity-related DM and its sequelae. Certain probiotic strains and combination of strains have a positive impact on gut bacteria which could help manage DM as well as ameliorate certain consequences [19,20]. The introduction of Lactobacillus reuteri GMNL-263 (contained in some probiotics) into the gut can enhance antioxidant effects against chronic inflammation and apoptosis of pancreatic beta cells; and lead to secretion of sufficient insulin thereby augmenting the established medical protocol in the management of DM. Also, complementary medicine alternatives may lessen dependency on certain drugs particularly those with notable adverse effects [21]. Including dietary fibers can aid in rebalancing the gut microbiome promoting healthy body weight and proper blood glucose levels.
Conflict of Interest Statement
The authors declare that this paper was written in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Acknowledgment
T.L. Brown (Harvard University) is acknowledged for his early contribution to this project.
References
- Zhang Y, Zhang H (2013) Microbiota associated with type 2 diabetes and its related complications. Food Science and Human Wellness 2(3-4): 167-172.
- Aitken JD, Gewirtz AT (2013) Toward understanding and manipulating the gut microbiota. Nature Reviews Gastroenterology & Hepatology 10(2): 72-74.
- Waldecker U (2016) Factors predicting a foot ulcer in the diabetic neuropathic foot. Foot and Ankle Surgery 22(2): 102.
- Elson C (2017) Faculty of 1000 evaluation for early pregnancy probiotic supplementation with lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomized controlled trial. F1000 - Post-publication peer review of the biomedical literature 11(4): 341-412.
- Butler G, Kirk J (2017) Diabetes mellitus. oxford medicine online 12(11): 163-181.
- Kavitha KM (2018) Knowledge and practice regarding foot Care among patients with type 2 diabetes mellitus. International Journal of Medicine and Pharmaceutical Sciences 8(1): 39-44.
- Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA (2012) The Neuropathic diabetic foot ulcer microbiome Is associated with Clinical Factors Diabetes 62(3): 923-930.
- Ventura M, Turroni F, Strati F, Sinderen DV (2015) The gut microbiota in health and disease. The human microbiota and microbiome 13(5): 136- 146.
- Woting A, Blaut M (2016) The intestinal microbiota in metabolic disease. Nutrients 8(4): 202.
- Lipsky BA, Richard J, Lavigne J (2013) Diabetic foot ulcer microbiome: one small step for molecular microbiology . . . one giant leap for understanding diabetic foot ulcers? Diabetes 62(3): 679-681. doi:10.2337/db12-1325
- Niafar M, Mofid V (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28(5): 539-543.
- Gruber L, Haller D (2014) Role of the Gut Microbiota in Maintaining GI Health: Highlights on Diabetes Mellitus. Molecular and Integrative Toxicology 9(7): 261-310.
- Huertas M, Michan C (2014) Gut Microbiota: In Sickness and in Health. Health and the Gut 11(3): 11-15.
- Shalem T, Weiss B (2015) Probiotic supplementation in patients with diabetes mellitus. Diet and Exercise in Diabetes Mellitus 6(9): 243-246.
- Nabavi S, Rafraf M, Somi M (2015) Probiotic yogurt improves body mass index and fasting insulin levels without affecting serum leptin and adiponectin levels in non-alcoholic fatty liver disease (NAFLD). Journal of Functional Foods 18: 684-691.
- Patterson E, Ryan PM, Cryan JF (2016) Gut microbiota, obesity, and diabetes. BMJ Journals. Postgraduate Medical Journal 92: 286-300.
- Keen H (2011) Epidemiologic Aspects of Diabetes Mellitus in Europe. The Epidemiology of Diabetes Mellitus 7(4): 11-120.
- Tungland B (2018) gut microbiota, early colonization and factors in its development that influence obesity and diabetes mellitus. Human Microbiota in Health and Disease 15(1): 1-35.
- Richardson AE, Kerna NA, Tulp OL (2018) Fundamental Factors Affecting the Development and Function of the Pediatric Microbiome. Arch Pediatr: JPED-157. DOI: 10.29011/2575-825X. 100057
- Kerna NA. A complementary medicine approach to augmenting antibiotic therapy: current practices in the use of probiotics during antibiotic therapy. Int J Complement Alt Med. 2018;11(2):62–66. DOI: 10.15406/ijcam.2018.11.00368
- Kerna NA. Global Health Preventive Medicine Overture: Select Probiotic use in the Prevention of Antibiotic-Associated Diarrhea and the Treatment of C. Difficile and Distinct Tropical Diseases. SM Prev Med Public Health. 2018; 2(3): 1021
© 2019 Nicholas A Kerna. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






