- Submissions

Full Text
Advances in Complementary & Alternative medicine
Exploration of the Sea Cucumber, Holothuria arenicola Health Benefits Holothuria arenicola as Promising Material for Therapeutic Drugs
Sohair R Fahmy*
Department of Zoology, Egypt
*Corresponding author: Sohair R Fahmy, Department of Zoology, Egypt
Submission: April 17, 2018;Published: January 11, 2019

ISSN: 2637-7802 Volume3 Issue4
Abstract
Marine invertebrates have become attractive as nutraceutical and functional foods and as a source material for the development of therapeutic drugs. The bioactivity of echinoderm, Holothuria arenicola extracts and their secondary metabolites has been identified by researchers as a promising potential rich source of natural drugs. H. arenicola is rich in several phenolic compounds, amino acids, alkaloids and other important nutraceutical compounds. The health benefits of H. arenicola are associated with its antioxidant, antifibrotic and antimicrobial effects. Thus, this review aims to elaborates and analyze the role of H. arenicola in the modulation of several organ-specific diseases.
Keywords: Holothuria arenicola; Anti-ulcerogenic; Antifibrotic; Anti-microbial; Antioxidants
Introduction
Marine invertebrates constitute one of the major groups of marine organisms from which a wide range of natural products have been devised [1]. However, there is increasing interest in the bioactivity of echinoderms extracts and their secondary metabolites. The consumption of sea cucumbers is thought to boost the immune system and to have aphrodisiac properties. They are gelatinous marine resources that are shaped like a cucumber that feed on microscopic algae, absorbing nutrients from the organic matter [2]. It is considered as “sea ginseng” because of its known medicinal properties aside from its nutritional value. The therapeutic use of the sea cucumbers for healing is established, where they were used for joint pain, tendonitis, and sprains [3]. Sea cucumber extracts usually showed multiple biological activities such as wound healing and antimicrobial, anticancer, and immunomodulatory [4,5]. They are also remarkably rich in vitamins, trace elements, and polysaccharides (chondroitin sulfate), which reduce arthritis pain, inhibit viral activities, and saponin glycosides that inhibit cancer activities [3].
Figure 1:External feature of Holothuria arenicoloa.

Holothuria arenicola Semper 1868 (Figure 1) is distinctive burrowing holothurians that are very common throughout the tropical regions of the world and it was one of the best known tropical shallow water species. The specific name arenicola Semper, 1868 is universally accepted, and has been used in a large number of systematic and ecological publications. In Egypt, Holothuria arenicola is distributed along the Alexandrian Mediterranean coast [6]. In 1984 H. arenicola was recorded for the first time on the Egyptian Mediterranean coast [7]. In 1998, small scale sea cucumber fishery began in Egypt in the southern part of the Red Sea. Later in the year of 2000, the sea cucumber fishery excelled greatly as a result of the high demand for the sea cucumber due to its consumption as a food newly introduced in Egypt and for exportation purposes. During those years Egypt has become one of the most important countries supplying the sea cucumber [8]. Sea cucumbers are well known to exert many beneficial effects on human health.
Nutritional Compounds of Holothuria arenicoloa
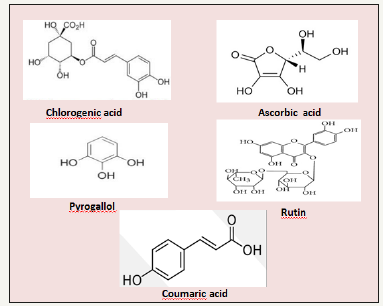
Sea cucumber is an attractive bioactive source due to the presence of several bioactive compounds with therapeutic properties. Nutrition content of the H. arenicola plays an essential function in its medicinal, nutritional, and therapeutic properties. High-performance liquid chromatography analysis of H. arenicola revealed the presence of five non-volatile phenolic compounds [9] (Figure 2). Chlorogenic acid was the major component (89.66%), whereas ascorbic acid (0.077%) was the minor component. Other components, such as pyrogallol (1.88%), rutin (1.06%) and coumaric acid (1.23%) were also recorded. Presence of active phenolic compounds in the body wall of the sea cucumbers, H. arenicola may be due to phenolic-rich materials such as phytoplankton and particles derived from degrading marine macroalgae which the main sources of food for sea cucumbers are [10]. Phenolic compounds are very important antioxidant compounds [11], due to their redox properties which play an important role as free radical scavengers, reducing agents, quenchers of singlet oxygen and complexes of pro-oxidant metals [12]. Feng et al. [13] has been reported that chlorogenic acid is one of the most abundant polyphenols in the human diet that has been known to decrease the incidence of chemical carcinogenesis in several animal models of cancer. It is an important component of coffee [14]. The major polyphenol in coffee is CGA. CGA is an ester formed from cinnamic acids and quinic acid and is also known as 5-O-caffeoylquinic acid (5-CQA) (IUPAC numbering) or 3-CQA (pre-IUPAC numbering) [15]. It was demonstrated that CGA exert many biological activities such as antibacterial, antioxidant, and anticarcinogenic activities, particularly hypoglycemic and hypolipidemic effects [16-19].
Figure 2:Chemical structure of the H. arenicoloa body wall phenolic compounds.
The potential hepatoprotective effect of chlorogenic acid in several animal models of liver injury was reported [9,12]. Chemical screening of the H. arenicola body wall and coelomic fluid showed presence of other important bioactive compounds which exhibit numerous medicinal benefits and health functions, especially flavonoids, alkaloids, tannins, quinones, saponins, proteins and amino acids [20]. The use of H. arenicola in the traditional medicine may originate from its ability to regenerate body wall after being cut up [21]. In addition, Bordbar et al. [22], reported that the health benefits of sea cucumbers are associated with the presence of bioactive components such as saponins, glycosaminoglycans, sterols, cerebrosides, peptides, sulfated polysaccharides, and essential fatty acids.
Antiulcerogenic effects of H. arenicola
Peptic ulcer is the most prevalent disease among the gastrointestinal diseases in most part of the world. Gastric ulcer develops because of several endogenous and exogenous factors [23]. It results as a consequence of impaired balance between gastro-protective factors such as mucus, bicarbonate and prostaglandins, and gastro-destructive substances [24]. The incidence varies with the age, gender, geographical location and is associated with severe complications including hemorrhages, perforations, gastrointestinal obstruction, and penetration [25]. Under normal conditions a large number of defense mechanisms prevent local damage and maintain structural and functional mucosal integrity [26]. Tradi tional medicines have been used to prevent or treat gastric ulcers for a long time. Accordingly, many natural products have been examined for their anti-ulcerogenic effects [27,28].
H. arenicola extract has been shown to produce promising antiulcerogenic efficacy that attributed to its high content of chlorogenic acid (CGA) [29]. Gastric ulcer lesion usually occurred due to increased gastric acidity secretion [30]. Most therapeutic agents exert their actions through inhibition of the gastric acid secretion [31]. H. arenicola induced marked improvement in gastric acid volume as compared to ranitidine (RAN) in rat model of gastric ulcer [29]. Gastric acid decimation by ranitidine is attributed to its ability to antagonize the binding of histamine to the H2 receptor on the parietal cells [32]. H. arenicola may be interferes with the gastric acid secretion and eliciting gastroprotection via an adaptive mechanism. These findings indicate that the H. arenicola probably acts by inhibiting H2-receptor leading to blockade of histamine release whose stimulatory action on gastric acid secretion via H2- receptor, has been well reported [33]. It has been reported that CGA exert inhibitory effect on mast cell activation and subsequently histamine release [34]. Thereby, H. arenicola may decrease gastric acid secretion due to inhibition of histamine secretion as a result of its high content of CGA.
Figure 3:Schematic diagram showing antiulcerogenic mechanism of H. arenicola body wall extract on some gastric indices.
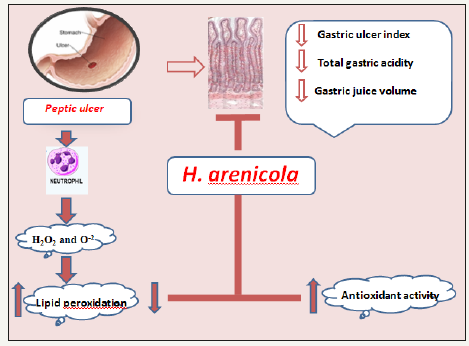
Gastric ulceration is a benign lesion on the mucosal epithelium upon exposure of the stomach to excess acid and aggressive pepsin activity [35]. Fahmy et al. [29] showed that H. arenicola exert significant improvement in gastric ulcer index as compared to the standard drug RAN (Figure 3). H. arenicola may stimulate gastric mucosal cellular growth and repair by the same mechanism of wound healing. Indeed, the damage of the gastric mucosa is also related to the increase in neutrophil infiltration into ulcerated tissues. These neutrophils inhibit ulcer healing mediated lipid peroxidation through the release of cytotoxic factors such as superoxide and hydrogen peroxide. It was reported that RAN caused significant inhibition of neutrophil activation and gastric acid secretion [36]. H. arenicola may stimulate gastric mucosal cellular growth via inhibition of neutrophil activation. This mechanism can be supported by the study of Shimoyama et al. [37] and Zatorski et al. [38], who reported that CGA treatment blocked the influx of neutrophils into the gastric tissue. Other studies have also shown that CGA inhibits neutrophil recruitment into inflammatory tissues [39,40].
Moreover, the anti-ulcerogenic effects of H. arenicola may be due to its antioxidant effect [29,41]. H. arenicola provided a marked suppression of oxidative damage through excellent radical scavenging activity to DPPH radical. Moreover, many dietary polyphenols are antioxidants, and the possibility exists that they protect against oxidative damage by directly neutralizing reactive oxidants [42]. The body wall of the sea cucumbers contains high amounts of phenolic compounds [43], which may exert scavenging activities by donating a hydrogen atom from their phenolic hydroxyl groups [11]. Thereby, H. arenicola may be an effective source of direct precursors for salvage glutathione reduced (GSH) biosynthesis.
The enhancement of the GSH level by the H. arenicola could be due to either its effect on the de novo synthesis of glutathione, its regeneration, or both [44]. Moreover, H. arenicola may act directly and scavenges the reactive oxygen species (ROS) derived by oxidation-reduction cycle with the cell or it may work in union with the existing antioxidant compounds and helps to prevent their loss during the ulcer oxidative injury. Fahmy et al. [29] also showed that H. arenicola induced significant enhancement in the activities of GST, CAT and SOD antioxidant enzymes to prevent the accumulation of excessive free radicals and protect stomach from ulcer formation, suggesting that the anti-ulcerogenic effect of H. arenicola against oxidative stress induced injury might be involved in decreasing lipid peroxide generation and stimulating antioxidant enzyme. Fahmy et al. [29] proved that H. arenicola may overpower gastric ulcer onslaught by suppressing the formation of ROS and protecting the antioxidant machinery. Moreover, the induction of the antioxidant enzymes by the H. arenicola represents a promising preventive strategy as a bifunctional inducer, along with the enhancement of antioxidant system enzymes which affords protection against cellular damage and inhibits ulcer promotion.
Antifibrotic effects of H. arenicola
Chronic liver disease is an important cause of morbidity and mortality and represents a major health problem worldwide [45]. The high prevalence of chronic liver diseases in Egypt has led to increasing numbers of Egyptian patients suffering from end-stage liver disease [46]. Obstructive jaundice, a frequently observed condition caused by obstruction of the common bile duct or its flow may end up with serious complications like hepatic failures [47]. Cholestatic liver fibrosis, characterized by excessive accumulation of extracellular matrix (ECM) proteins [48]. Cholestasis represents the consequence of impaired bile formation and generally caused by conditions that the enterohepatic circulation is interrupted, and bile acids accumulate within the liver [49]. Mainly inflammatory cell infiltration, hepatocyte necrosis, and liver fibrosis are the main pathological features of cholestasis [50]. Retention of hydrophobic bile salts within the hepatocytes during cholestasis leads to apoptosis and necrosis [51]. Oxidative stress (OXS) and inflammatory injuries are the most important pathogenic events in cholestatic liver injury [9,52].
Liver fibrosis, which etiologically and pathogenetically resembles the biliary fibrosis in the human beings can be induced by bile duct ligation and [53]. Several potential therapies for fibrosis have been identified in previous preclinical studies. These include interruption of matrix deposition and hence inhibition of collagen synthesis [54]. For the therapeutic strategies of liver injury and disease, it is important to find antioxidant compound that are able to block liver injuries through free radicals generated due to toxic chemicals. During the course of evolution, many invertebrates have established as a selective advantage by endogenous production of protective chemicals [55]. It was demonstrated that H. arenicola exerted an apparent arrest in the progression of collagen deposition in the cholestatic animals which may be a consequence of the increased mass of regenerated liver cells [9] This give an additional support that H. arenicola are able to condition the hepatocytes, accelerate regeneration of parenchyma cells, protect against membrane fragility and hence decrease leakage of the enzymes into circulation.
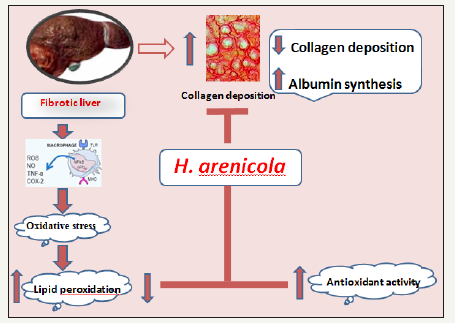
Acceleration in albumin synthesis may be another mechanism by which H. arenicola extract exert its antifibrotic efficacy [9]. Awang [56] has been reported that stimulation of albumin synthesis has been advanced as a contributory hepatoprotective mechanism which accelerates the regeneration process and the production of liver cells [56]. It was reported that oxidative stress occurs during cholestasis plays a role in cholestasis induced liver fibrosis [57]. Tissues and cells would be subjected to oxidative injuries when large quantities of inner free radicals are generated, or the activities of antioxidant system deteriorate. Accordingly, antioxidant therapy represents a potential strategy to prevent liver injury and fibrosis. Treatment with H. arenicola normalized the antioxidant levels during cholestasis through its rich of polyphenolic compounds especially chlorogenic acid that has the ability to scavenge free radicals. Wang et al. [58] proved that chlorogenic acid decreased malondialdehyde (MDA) contents in liver while increased activities of antioxidant enzymes (Figure 4).
Figure 4:Schematic diagram showing antifibrotic mechanism of H. arenicola body wall extract.
Antimicrobial effects of H. arenicola
Several drug discovery projects have screened for echinoderms for antibiotic activities. Moreover, anti-fungal, anti-bacterial, antithrombotic, anti-malarial, anti-protozoa and anti-virus effects have been reported from some sea cucumber isolated compounds [59]. Many investigators reported the in vitro antibacterial and antifungal activities of Holothuria leucospilota body wall and coelomic fluid [60,61]. Fahmy et al. [20] proved the Body wall extract of the H. arenicola showed a broad-spectrum antibacterial activity against Staphylococcus aureus and Escherichia coli. The antibacterial potency of the H. arenicola may be due to their antimicrobial components, flavonoids, alkaloids, tannins, quinines and saponins. It was reported that these active constituents have antibacterial characteristics [62].
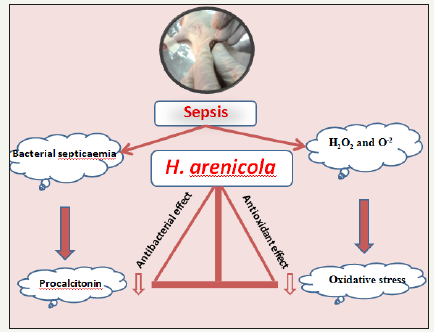
Sepsis, a systemic inflammatory response syndrome (SIRS) induced by infection, is accompanied by the presence of bacteria [63]. Abundant evidence shows that CLP-induced sepsis with acute supportive peritonitis is a typical sepsis model with G-bacteria as the predominant infection source [64]. In a study by Fahmy et al. [20], the antimicrobial effects of H. arenicola was examined using rat model for cercal ligation and puncture (CLP) in which 200mg/kgb.wt. of the methanolic extract of H. arenicola was used. The results showed that H. arenicola body wall extract successfully increased survival rate of septic rats to 66.7% through its antibacterial activity. Procalcitonin (PCT), the precursor for the hormone calcitonin (CT) is a biomarker that exhibits greater specificity than other proinflammatory markers (cytokines) in identifying patients with sepsis and can be used in the diagnosis of bacterial infections [65] (Figure 5). Usually, PCT used to guide antibacterial therapy as a surrogate biomarker [66]. Fahmy et al. [20] showed that treatment of septic rats with H. arenicola body wall extract succeed to normalize the PCT level which may be due to their antimicrobial constituents. Accordingly, antibacterial of H. arenicola body wall extract may be due to its content of polyphenolic compound especially quinine, saponins and terpenoid that show antibacterial characteristics [20].
Figure 5:Schematic diagram showing antiseptic mechanism of H. arenicola body wall extract.
References
- Jimenez JT, Sturdikova M, Studik E (2009) Natural products of marine origin and their perspectives in the discovery of new anticancer drugs. Acta Chimica Slovaca 2(2): 63-74.
- Conand C (1990) The fishery resources of pacific island countries Part 2: Holothurians. FAO Fisheries Technical Paper 272.2; Food and Agriculture Organization of the United Nations, Rome, Italy, p. 143.
- Hamel JF, Mercier A (2004) Synchronous gamete maturation and reliable spawning induction method in holothurians. In: Lovatelli, Conand C, Purcell S, Uthicke S, Hamel JF, et al. (Eds.), Advances in sea cucumber aquaculture and management. FAO Fisheries Technical Reports No. 463, FAO, Rome, Italy.
- Ridzwan BH, Kaswandi MA, Azman Y, Fuad M (1995) Screening for antibacterial agents in three species of sea cucumbers from coastal areas of Sabah. Gen Pharmacol 26(7): 1539-1543.
- Tian F, Zhang X, Tong Y, Yi Y, Zhang S, et al. (2005) PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo. Cancer Biol Ther 4(8): 874-882.
- Abdel RF, Abdel R, Moussa RM, Mena MH (2012) Captive spawning of Holothuria arenicola (semper, 1868) from Egyptian Mediterranean coast. Asian Journal of Biological Sciences 5(8): 425-431.
- Shoukr FA, Mona MH, Abdel Hamid ME (1984) Holothurians (Echinodermata: Holothuroidea) from some Egyptian shores. Bulletin of the Faculty of Sciences of the Zagazig University 6: 662-682.
- Ahmed MI (2006) Taxonomic and fishery stock status of sea cucumber in the Egyptian Red Sea. M. Phil Thesis Hull University, UK.
- Fahmy SR (2015) Anti-fibrotic effect of Holothuria arenicola extract against bile duct ligation in rats. BMC Complementary and Alternative Medicine 15: 14.
- Althunibat OY, Hashim RB, Taher M, Daud JM, Ikeda MA, et al. (2009) In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. European Journal of Scientific Research 37: 376-387.
- Mustafa RA, Abdul HA, Mohamed S, Bakar FA (2010) Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. Journal of Food Science 75(1): 28-35.
- Xu Y, Chen J, Yu X, Tao W, Jiang F, et al. (2010) Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm Res 59(10): 871-877.
- Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, et al. (2005) Inhibition of activator protein-1, NF-B, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem 280(30): 27888-27895.
- Olthof MR, Hollman PCH, Katan MB (2001) Chlorogenic acid and caffeic acid are absorbed in humans. Journal of Nutrition 131(1): 66-71.
- Clifford MN (2000) Chlorogenic acids and other cinnamates-nature occurrence dietary burden absorption and metabolism. Journal of the Science of Food and Agriculture 80(7): 1033-1043.
- Kono Y, Kobayashi K, Tagawa S, Adachi K, Ueda A, et al. (1997) Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochimica et Biophysica Acta-General Subjects 1335(6): 335- 342.
- Kasai H, Fukada S, Yamaizumi Z, Sugie S, Mori H (2000) Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food and Chemical Toxicology 38(5): 467-471.
- Dos Santos MD, Almeida MC, Lopes NP, de Souza GE (2006) Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biological and Pharmaceutical Bulletin 29(11): 2236-2240.
- Bassoli BK, Cassolla P, Borba MGR, Constantin J, Salgueiro PCL, et al. (2008) Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell Biochemistry and Function 26(3): 320-328.
- Fahmy SR, Soliman AM, Sayed AA, Abd Al, Shakour MY (2015) Holothuria arenicola as a new antiseptic drug: In vitro antibacterial investigation and in vivo therapeutic role. Research Journal of Pharmaceutical, Biological and Chemical Sciences 6(5): 366- 374.
- Omran (2013) NEE: Nutritional value of some Egyptian sea cucumber. African J Biotechnol 12(35): 5466-5472.
- Bordbar S, Anwar F, Saari N (2011) High-value components and bioactive from sea cucumbers for functional foods. Mar Drugs 9(10): 1761-1805.
- Nartey ET, Ofosuhene M, Agbale, CM (2012) Anti-ulcerogenic activity of the root bark extract of the African laburnum Cassia sieberiana and its effect on the anti-oxidant defence system in rats. BMC Complement Altern Med 12: 247.
- Tytgat GN (2011) Etiopathogenetic principles and peptic ulcer disease classification. Dig Dis 29(5): 454-458.
- Helpern GM (2004) Natures safe and effective remedy for ulcers. Square one publisher, Inc consumers health, pp. 1-165.
- Tulassay Z, Herszényi L (2010) Gastric mucosal defense and cytoprotection. Best Practice & Research Clinical Gastroenterology 24(2): 99-108.
- Ozbayer C, Kurt H, Ozdemir Z, Tuncel T, Moheb SS, et al. (2013) Gastroprotective, cytoprotective and antioxidant effects of oleum cinnamomi on ethanol induced damage. Cytotechnology.
- Choudhary M, Kumar V, Singh S (2014) Gastric antisecretory an cytoprotective effects of hydroalcoholic extracts of Plumeria alba linn leaves in rats. J Integr Med 12(1): 42-51.
- Fahmy SR, Amer MA, Al killidar MH (2015) Ameliorative effect of the sea cucumber Holothuria arenicola extract against gastric ulcer in rats. The Journal of Basic & Applied Zoology 72: 16-25.
- Nagar H, Tiwari P, Jain DK, Chandel HS (2012) Evaluation of anti-ulcer activity of stem bark extract of Aphanmixis polystachya in experimental Rat Ind J Pharm Edu Res 46(3): 222-227.
- Tiwari DK, Tripathi RK, Jena J (2012) evaluation of antiulcer and antianxiety activity of Aphanamixis polystachya stem bark extract on rats int. J Pharm Pharm Sci 5: 148-151.
- Banji D, Singh J, Banji OJ (2010) Scrutinizing the aqueous extract of leaves of pedalium murex for the antiulcer activity in rats. Pak J Pharm Sci 23(3): 295-299.
- Dial E, Thompson J, Rosenfeld G (1981) Isolated parietal cells histamine response and pharmacology. J Pharmacol Exp Therap 219(3): 586-590.
- Qin HD, Shi YQ, Liu ZH, Li ZG, Wang HS, et al. (2010) Effect of chlorogenic acid on mast cell-dependent anaphylactic reaction. Int Immunopharmacol 10(9): 1135-1141.
- Sabiu S, Garuba T, Sunmonu T, Ajani E, Sulyman A, et al. (2015) Indomethacin-induced gastric ulceration in rats: protective roles of spondias mombin and ficus exasperate. Toxicology Reports 2: 261-267.
- Okajima K, Murakami K, Liu W, Uchiba M (2000) Inhibition of neutrophil activation by ranitidine contributes to prevent stress-induced gastric mucosal injury in rats. Crit Care Med 28(8): 2858-2865.
- Shimoyama AT, Santin JR, Machado ID, De Oliveira e SAM, De Melo IL, et al. (2013) Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn Schmiedebergs Arch Pharmacol 386(1): 5-14.
- Zatorski H, Sałaga M, Zielińska M, Piechota P, Owczarek K, et al. (2015) Experimental colitis in mice is attenuated by topical administration of chlorogenic acid. Naunyn Schmiedebergs Arch Pharmacol 388(6): 643- 651.
- Kim JH, Kim BW, Kwon HJ, Nam SW (2011) Curative effect of selenium against indomethacin-induced gastric ulcers in rats. Journal of Microbiology and Biotechnology 21: 400-404.
- Zhang Y, Song S, Liang H, Wang Y, Wang W, et al. (2010) enhancing effect of a sea cucumber Stichopus japonicas sulfated polysaccharide on neurosphere formation in vitro. Journal of Bioscience and Bioengineering 110(4): 479-486.
- Ardestani SK, Janlow MM, Kariminia A, Tavakoli Z (2004) Effect of cimetidine and ranitidine on lipid profile and lipid peroxidation in γ-irradiated mice. Acta Med Iran 42: 198-204.
- Moskaug, JØ, Carlsen H, Myhrstad MCW, Blomhoff R (2005) Polyphenols and glutathione synthesis regulation. Am J Clin Nutr 81: 277S-283S.
- Althunibat OS, Hashim RB, Taher M, Daud JM, Ikeda M (2009) In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur J Sci Res 37: 376–387
- Park SW, Lee CH, Kim YS, Kang SS, Jeon SJ, et al. (2008) Protective effect of baicalin against carbon tetrachloride-induced acute hepatic injury in mice. Journal of Pharmacological Sciences 106(1): 136-143.
- Bessa SS, Mohamed Ali EM, Abd El Wahab AE, Nor El Din SA (2012) Heme oxygenase-1 mrna expression in Egyptian patients with chronic liver disease. Hepat Mon 12(4): 278-285.
- Awadallah AM, Issa HA, Soliman MS (2011) evaluation of serum chromogranin a as a useful tumor marker for diagnosis of hepatocellular carcinoma. Journal of American Science. 7: 999-1007.
- Aydın S, Tokaç M, Taner G, Arıkök AT, Dündar, et al. (2013) Antioxidant and antigenotoxic effects of lycopene in obstructive jaundice. J Surg Res 182(2): 285-295.
- Han JM, Kim HG, Choi MK, Lee JS, Park HJ, et al (2012) Aqueous extract of artemisia iwayomogi kitamura attenuates cholestatic liver fibrosis in a rat model of bile duct ligation. Food Chem Toxicol 50(10): 3505-3513.
- Jüngst C, Berg T, Cheng J, Green RM, Jia J, et al. (2013) Intrahepatic cholestasis in common chronic liver diseases. Eur J Clin Invest 43(10): 1069-1083.
- Hofmann AF (2002) Cholestatic liver disease: Pathophysiology and therapeutic options. Liver Suppl 2: 14-19.
- Lee TY, Chang HH, Chen JH, Hsueh ML, Kuo JJ (2007) Herb medicine ameliorates hepatic fibrosis in bile duct ligation rats. J Ethnopharmacol 109(2): 318-324.
- Jin F, Cheng D, Tao JY, Zhang SL, Pang R, et al. (2013) Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol 13: 79.
- Nasehi M, Tackallou SH, Hasani I, Nasehi M (2013) Cholestasis impaired spatial and non-spatial novelty detection in mice. Journal of Paramedical Sciences 4: 92-98.
- Fuentes BL, Miana MFJ, Piedrafita E, Berzosa C, Martínez BE, et al. (2010) Melatonin protects against taurolithocholic- induced oxidative stress in rat liver. J Cell Biochem 110(5): 1219-1225.
- Fahmy SR, Hamdi SA (2011) Curative effect of the Egyptian marine erugosquilla massavensis extract on carbon tetrachloride-induced oxidative stress in rat liver and erythrocytes. Eur Rev Med Pharmacol Sci 15(3): 303-312.
- Awang D (1993) Milk thistle. Can Pharm J, pp. 749-754.
- Pastor A, Collado PS, Almar M, Gonzalez G J (1997) Antioxidant enzyme status in biliary obstructed rats: Effects of N-acetylcysteine. J Hepatol 27(2): 363-370.
- Wang JH, Liu YL, Li CL, Yu J, Wang F (2012) Effect of chlorogenic acid extracted from Eucommia ulmoidesoliv on hyperlipemia of mice induced by high fat diet. Science and Technology of Food Industry 15: 360-362.
- Kumar R, Chaturvedi AK, Shukla PK, Lakshmi V (2007) Antifungal activity in triterpene glycosides from the sea cucumber actinopyga lecanora. Bioorg Med Chem Lett 17(15): 4387-4391.
- Adibpour N, Nasr F, Nematpour F, Shakouri A, Ameri A (2014) Antibacterial and antifungal activity of Holothuria leucospilota isolated from Persian Gulf and Oman Sea. Jundishapur J Microbiol 7(1).
- Shakouri A, Nematpour F, Adibpour N, Ameri A (2014) Environmental studies of Persian Gulf. Environmental studies of Persian Gulf 7: 135- 140.
- Dhinakaran DI, Lipton AP (2014) Bioactive compounds from Holothuria atra of Indian ocean. International Journal of Biology and Biological Sciences 3: 673.
- Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH (2003) Screening of medicinal plant extracts for antioxidant activity. Life Sci 73(2): 167-179.
- Seely KA, Holthoff JH, Burns ST, Wang Z, Thakali KM (2011) Changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 301(1): F209-F217.
- Harrison M, Collins CD (2015) Is Procalcitonin-guided antimicrobial use cost-effective in adult patients with suspected bacterial infection and sepsis. Infect Control Hosp Epidemiol 36(3): 265-272.
- Li H, Luo YF, Blackwell TS, Xie CM (2011) Meta-analysis and systematic review of procalcitonin-guided therapy in respiratory tract infections. Antimicrob Agents Chemother 55(12): 5900-5906.
© 2018 Sohair R Fahmy. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






