- Submissions

Full Text
Advances in Complementary &Alternative Medicine
Antidiabetic Potential of Eugenia jambolana Ethanolic Seed Extract: Effect on Antihyperlipidemic and Antioxidant in Experimental Streptozotocin-Induced Diabetic Rats
Sunil Mahajan1, Pratibha Chauhan1, Meerambika Mishra1, Dhananjay Yadav3, Mousumi Debnath2 and GBKS Prasad1*
1Jiwaji University, India
2Department of Biosciences, Manipal University Jaipur,, India
3Department of Medical Biotechnology, Yeungnam University, South Korea
*Corresponding author: GBKS Prasad, Centre for Translational Research, School of Studies in Biochemistry, Jiwaji University, Gwalior-474011, India
Submission: April 03, 2018; Published: April 25, 2018

ISSN: 2637-7802Volume2 Issue3
Abstract
Background: The present study investigated antihyperglycemic, and antioxidant potentials of Eugenia jambolana seed in streptozotocin (STZ)- induced diabetic rats.
Methods: Male Wistar rats were made diabetic by a single dose of Streptozotocin (45mg/kg body weight .i.p). The animals were administered of ethanolic extract of Eugenia jambolana (E. jambolana) seed at the dose of (250mg/kg body weight) orally. The therapeutic functions of the E. jambolana was assessed by monitoring, Blood glucose levels and body weight of rats were measured at weekly intervals and glycosylated hemoglobin, lipid profile and biomarkers of oxidative stress, liver and kidney function markers and degree of DNA damage were also measured at the end of the study.
Results: Daily administration of E. jambolana seed extract for 28 days resulted in significant reductions of blood glucose and glycosylated hemoglobin levels. There was also a significant increase in HDL-Cholesterol levels with concomitant decreases in total cholesterol, triglycerides, LDL-Cholesterol, VLDL. Also, a significant improvement in enzymatic and non-enzymatic biochemical markers of oxidative stress. The kidney and liver functions were also reverted back to near normalcy by E. jambolana ethanolic seed extract.
Conclusion: The present results showed the anti-hyperglycemic, and anti-oxidative potential of of ethanolic extract of E. jambolana in STZ induced diabetic rats. It was concluded that the ethanolic extract of E. jambolana is potent in regulating not only hyperglycemia but also hyperlipidemia and oxidative stress in STZ induced diabetic rats.
Keywords: Diabetes mellitus; Eugenia jambolana; Oxidative stress; Blood glucose; Antihyperlipidemic; Antioxidant
Abbreviations: E. jambolana: Eugenia jambolana; BW: Body Weight; GSH: Reduced glutathione; HbA1c: Glycosylated Haemoglobin; GPx: Glutathione Perosidase; GR: Glutathione Reductase; GST: Glutathione-S Transferase; HDL: High-Density Lipoprotein cholesterol; LDL: Low-Density Lipoprotein cholesterol; SOD: Super Oxide Dismutase; AST: Aspartate Transaminase; ALT: Alanine Transaminase; VLDL: Very Low Density Lipoprotein cholesterol; TBARS: Thiobarbituric Acid Reactive Substances
Introduction
Diabetes is one of the chronic diseases characterized by hyperglycemia resulting from impaired insulin secretion, insulin action, or both and presently one of the vital causes leading to mortality and morbidity [1]. Chronic hyperglycemia is damaging to β-cells and peripheral tissues, a condition termed glucotoxicity, which is clinically related as a cause of diabetes-related complications such as cardiovascular disease, nephropathy, retinal blindness, neuropathy and peripheral gangrene [2,3]. Therefore, maintenance of glycemic homeostasis is the most common therapeutic aim for patients with Type 2 diabetes, the most prevalent type of diabetes. Moreover, abnormal lipid metabolism in adipose and other tissues can cause lipotoxicity, exhibit increased low density lipoprotein (LDL) and decreased high density lipoprotein (HDL) cholesterol levels and hypertension as well as altered platelet function [4]. Oxidative stress induced by reactive oxygen species (ROS) and nitrogen species produced by several biochemical pathways associated with hyperglycemia (glucose autooxidation, polyol pathway and protein glycation) is critically involved in the impairment of 𝛽-cell function during the development of type 2 diabetes [5]. According to a recently published report, a worldwide estimate of nearly 415 million diabetic patients and 193 million undiagnosed diabetes clearly depict the increasing rise of the disease globally [6].
There are several types of glucose-lowering drugs, including insulin and various oral anti-diabetic agents such as sulfonylureas, biguanides, Thiazolidinediones, α-Glucosidase inhibitors Meglitiridesare available [7-9], and these drugs are used as monotherapy or in combination to achieve better glycemic control. Each of the above oral antidiabetic agents are associated with moderate to serious adverse effects such as severe hypoglycemia, lactic acidosis, idiosyncratic liver cell injury, digestive discomfort, headache, dizziness and even death [10]. Also, there are evidences that these drugs become refractory in many cases over a period of time.
In India, indigenous herbal remedies such as Ayurveda and other Indian traditional medicine have since ancient times used plants in treatment of diabetes [11]. Some of the plants which are being used for the treatment of diabetes have received scientific or medicinal scrutiny and even the World Health Organization expert committee on diabetes recommends the evaluation and mechanistic properties of the plants effective in such systems [12]. More than 400 plants which have been reported to show antidiabetic potential [13-15]. A extensive collection of plant-derived active principles representing numerous bioactive compounds have established their role for possible use in the treatment of diabetes [16-18].
The E. jambolana which belongs to the family Myrtaceae, commonly known as black plumor Jamun, is being widely used to treat diabetes by the traditional practitioners over many centuries [19-21]. It is a large evergreen tree growing up to 30m high found widely in India. It is also found in Thailand and Philippines. Its fruits are oval to elliptical 1.5-3.5cm long, dark purple or nearly black, luscious, fleshy and edible [19]. The extract of E. jambolana pulp showed the hypoglycemic activity in streptozotocin induced diabetic mice within 30min of administration while the seed of the same fruit required 24h [22]. The antihyperglycemic activity of seeds of E. jambolana is well documented [23,24]. Sharma et al. [25] reported a decrease in the blood sugar level in alloxan diabetic rats by the intake of the ethanolic extract of E. jambolana The flavonoid rich E. jambolana seed extract when administered orally to experimental animals in various dose levels were found to be both hypglycemicand hypolipidemic [20,25], and shows profound effect on the carbohydrate and lipid metabolism on diabetic rats. Arun et al. [26] reported the use of aqueous and ethanolic extracts of E. jambolana showed significant protective effects against hydroxyl radical induced strand breaks in pBR322 DNA by some genotoxic carcinogens. The study of the antioxidant activity of the flavonoid rich E. jambolana fruit showed a significant correlation between the concentration of the extract and percentage inhibition of free radicals or percentage inhibition of lipid peroxidation [27]. The present study was undertaken to evaluate the anti-diabetic and anti-oxidant potential of ethanolic seed extract of E. jambolana in streptozotocin-induced diabetic male Wistar rats.
Materials and Methods
Plant material
Seed powder of E. jambolana was provided by M/S Dindayal Industries Pvt. Ltd. Gwalior Madhya Pradesh, India.
Preparation of ethanolic extract
E. jambolana powder was soaked in 95% ethanol for 24h with continuous stirring using magnetic stirrer. The resulting extract was filtered and the filtrate was evaporated under reduced pressure in Rotary evaporator. The resulting powder was used for experiment.
Drug and doses
The extract reconstituted in 0.5% dimethyl sulfoxide (250mg/ kg bw) and glibenclamide in normal saline (600μg/kg bw) was used as standard drug and administered orally for 28 days. The control group received normal saline orally.
Experimental animals and induction of diabetes
For this experiment, male Wistar rats weighing approximately 180-200g were procured from Defence Research and Development Establishment (DRDE), Gwalior, India. All the animals were acclimatized in department animal house under standard laboratory conditions (25-30 °C and at 45-55% relative humidity for 12 hours each of light and dark cycle). The animals were fed on standard pellet diet and water ad libitum. The rats used in the present study were maintained in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSE) regulations and guide for the care and use of laboratory animals. Diabetes was induced into overnight fasted rats by single intraperitoneal injection of freshly prepared Streptozotocin (45mg/ kg body weight) in citrate buffer (pH 4.5, 0.1M). Hyperglycemia or increased blood glucose was confirmed by checking the blood glucose by tail vein blood glucose levels using ACCU-CHEK sensor glucometer at 48 hours after streptozotocin injection. Rats showing hyperglycemia with blood glucose 200mg/dL were considered diabetic [28] and were used in the present study.
Experimental design
In this experiment, a total of twenty four rats were randomly divided into four groups of 6 each and were treated daily for 28 days as follows. The experimental period was 28 days beginning after the induction of STZ diabetes.
- Group I (normal control rats treated with distilled water)
- Group II (diabetic control rats treated with distilled water)
- Group III (diabetic rats administered daily dose of ethanolic extract of E. jambolana (250mg/kg b.w.)
- Group IV (diabetic rats administered daily with Glibenclamideat 600μg/Kg b.w.)
Collection and processing of blood and tissue samples
At the end of the experimental, blood was collected with anticoagulant and stored at -20 °C till used for estimation of biochemical parameters. The animals were sacrificed, the abdomen and thorax were opened, kidney, liver, pancreas and brain were removed and washed three times in ice cold saline and blotted individuallyin filter-paper, used for the preparation of tissue homogenates for estimation of glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST).
Preparation of tissue homogenate
The tissues were weighed, 10%w/v tissue homogenates were prepared and homogenizing the tissue in phosphate buffer (pH 7.5, 0.1M) separately. After centrifugation (10,000rpm at 4 °C for 10min), the clear supernatants were used for the estimation of biochemical variables.
Biochemical parameters
Fasting blood glucose estimation was done weekly after diabetes induction on tail prick blood of the overnight fasted rats and blood glucose was measured using ACCU-CHEK sensor glucometer. It uses glucose oxidase specific strips and works on principle of Reflectance Photometry. The fasting blood sugar levels of the normal control groups were also measured simultaneously. All the results were expressed in milligrams per deciliter (mg/dL) of the blood.
The blood HbA1C was measured after hemolysis of the anticoagulated whole blood specimen. HbA1C was determined by Ion exchange resin method [29]. The lipid profile parameters such as total cholesterol (CHOD-PAP method) [30], serum triglyceride (GPO-PAP Method [31], serum HDL-cholesterol (PEG/CHOD-PAP; precipitation Method [32], low density lipoprotein (LDL) and very low density lipoprotein (VLDL calculated from Freidewald’s Formula) were estimated. The kidney function markers such as serum creatinine (modified Jaffe’s Kinetic method [33], serum urea (modified Berthelot method [34] and serum uric acid Uricase/ PAP method [35] was done. Liver function markers such as serum glutamate pyruvate transaminase (SGPT or alanine transaminase) and serum glutamate oxaloacetate transaminase (SGOT or AST) by the modified International Federation of Clinical Chemistry method [36] and bilirubin [37] were estimated by using commercially available standard kits manufactured by Crest Biosystems, Pvt. Ltd. India.
Oxidative stress markers in blood
Oxidative stress markers like glutathione (reduced) (GSH); [38], superoxide dismutase (SOD) [39], Thiobarbituric acid reacting substance(TBARS); [40] and Catalase [41], were estimated in normal, diabetic and treated groups as per the reported methods . GSH was estimated in whole blood (50μl blood in 950μl Distilled water), TBARS, SOD and Catalase were estimated in hemolysate, and protein was estimated by the method of Lowry et al. [42]. All the parameters were expressed in terms of units/mg protein of the tissue. DNA damage was evaluated by Comet assay [43].
Oxidative stress markers in Liver and Kidney tissues
The activity of GPx was measured using a coupled enzyme assay as described by [44]. The decrease in absorbance was monitored at 25 °C at 340nm. One unit of enzyme activity was defined as μmol of GSH consumed/min/mg protein. The GR activity was measured in the soluble tissue extracts by the method of [45]. The decrease in absorbance was monitored at 25 °C at 340nm. One unit of enzyme activity is defined as μmol of NADPH oxidized/min/mg protein. GST activity was measured spectrophotometrically by the method described by [46], One unit of GST activity is defined as the amount producing/mmol of CDNB-GSH conjugate/min under the condition of the assay.
Statistical Analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test using Sigma stat 3.5. A value of p˂0.05 was considered significant and results are expressed as mean±SEM for six animals in each group.
Result
Effect of ethanolic seed extract of E. jambolana on hyperglycemia and body weight
Table 1 shows the levels of fasting blood glucose (FBG) in normal and diabetic rats at the time of diabetes induction as well as after 28 days of treatment. Streptozotocin (STZ) induction resulted in a significant increase in blood glucose level as compared to Group I (normal control group). The administration of ethanolic seed extract E. jambolana to diabetic rats resulted in significant reduction (54.5%) in the level of fasting blood glucose. After 28 days, the group III rats showed slightreduction (7.1%) in the level of glycosylated haemoglobin levels when compared to group II, the diabetic control. Body weight was decreased (13%) in Sinduced Group II, diabetic rats with respect to control on the 28th day. After the administration of E. jambolana seed extracts in Group III rats and glibenclamide treatment in group IV rats significantly reduced the blood sugar and glycosylated heamoglobin levels that was close to the Group I normal control rats (Table 1).
Table 1: Effect of ethanolic seed extract of Eugenia jambolana on hyperglycemia.
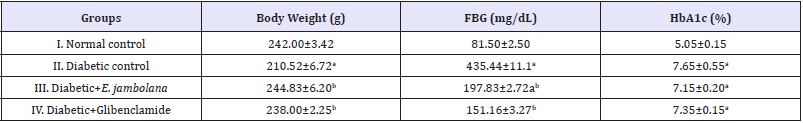
Data are expressed as mean±SEM of 6 animals in each group and significantly different (p< 0.05): ain comparison to normal control; bin comparison to diabetic control group. FBG: Fasting Blood Glucose; HbA1c: Glycosylated Haemoglobin
Effect of ethanolic seed extract of E. jambolana on lipidemia
The plasma TC and The TG was elevated significantly in the Group II, diabetic induced rats by 29.7% and 38.9% respectively in comparison the Group I normal control rats. Administration of E. jambolana for 28 days to the Group III diabetic rats resulted in reduction by 8.7% and 23.1% respectively in comparison to Group II Diabetic control rats Here was a marked difference in the levels of serum TC, TG, HDL, LDL and VLDL-cholesterol in Group I normal control and Group II diabetes control groups of rats (Table 2). The levels of LDL and VLDL-cholesterol were significantly (p<0.05) increased, whereas the HDL cholesterol was markedly (p<0.05) decreased (64.7%) in rats induced with STZ, when compared with Group II control rats. The diabetic rats treated with E. jambolana (Group III) and glibenclamide (Group IV) for 28 days, there was also a significant increase (63.7% and 70%) in HDL-cholesterol levels with concomitant decreases in all the other parameters respectively.
Table 2: Effect of ethanolic seed extract of Eugenia jambolana on lipidemia.
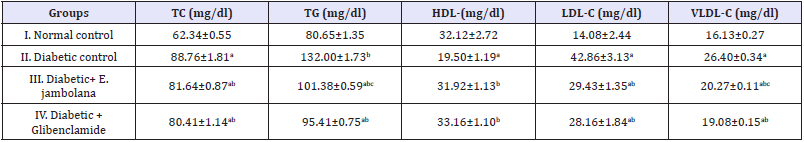
Data are expressed as mean±SEM of 6 animals in each group and significantly different (p<0.05): ain comparison to normal control; bin comparison to diabetic control group; and cin comparison to the Glibenclamide treated group. TC: Total Cholesterol; TG: Triglycerides; HDL: High Density Lipoprotein cholesterol; LDL: Low Density Lipoprotein Cholesterol; VLDL: Very Density Lipoprotein Cholesterol.
Effect of ethanolic seed extract of E. jambolana on biomarkers of toxicity
Table 3 shows the levels of serum urea, uric acid and creatinine, in normal control and diabetes control groups of rats showed a marked difference (Table 3). The levels of urea, uric acid and creatinine were significantly (p0.05) increased by 106.3%, 76.2% and 83.5% respectively when the rats of group II were induced with STZ. In the diabetic rats treated with E. jambolana extract (Group III) and glibenclamide (Group IV) for 28 days, significant (p<0.05) reduction in the level of urea, uric acid and creatinine respectively by 13.4%, 27% and 43.1% (Group III) 17.2%, 33.6% and 36.4% (Group IV) was noted. Thus there was no toxicity found in the kidney function markers. The levels of SGOT, SGPT and bilirubin were significantly (p<0.05) increased when, the rats of Group II were induced with STZ. The diabetic rats on treatment with E. jambolana extract (Group III) and glibenclamide (Group IV) for 28 days showed, significant (p<0.05) reduction by 23%, 20.5% in the level of SGOT, 30.9 %, 26.6 % SGPT and 15.9% 28.1% bilirubin. Thus there was no toxicity found in the liver function markers (Table 4).
Table 3: Effect of ethanolic seed extract of Eugenia jambolana on biomarkers of toxicity related to kidney function.

Data are expressed as mean±SEM of 6 animals in each group and significantly different (p<0.05): ain comparison to normal control; bin comparison to diabetic control group; and cin comparison to the Glibenclamide treated group.
Table 4: Effect of ethanolic seed extract of Eugenia jambolana on biomarkers of toxicity related to Liver function.
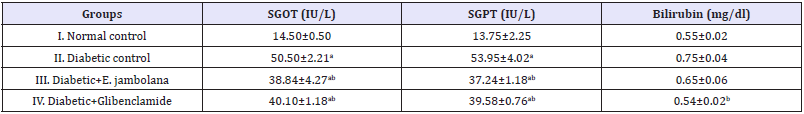
Data are expressed as mean±SEM of 6 animals in each group and significantly different (p<0.05): ain comparison to normal control; bin comparison to diabetic control group; and cin comparison to the Glibenclamide treated group.
Data are expressed as mean±SEM of 6 animals in each group and significantly different (p<0.05): ain comparison to normal control; bin comparison to diabetic control group; and cin comparison to the Glibenclamide treated group. SGOT: Serum Glutamate Oxaloacetate Transaminase; SGPT: Serum Glutamate Pyruvate Transaminase
Effect of ethanolic seed extract of E. jambolana on biomarkers of oxidative stress in blood
The biomarkers for oxidative stress, GSH, SOD catalase and TBARS in normal control and diabetes control groups of rats were studied. The levels of GSH, SOD and catalase were significantly (p<0.05) decreased when the rats were induced with STZ (Group II) while the level of TBARS showed a marked increased. The diabetic rats treated with extract (Group III) and glibenclamide (Group IV) for 28 days exhibited a significant (p<0.05) increase in the level of GSH, SOD, and catalase respectively, and concomitant decrease were observed in the level of TBARS (Table 5).
Table 5: Effect of ethanolic seed extract of Eugenia jambolana on biomarkers of oxidative stress in blood.
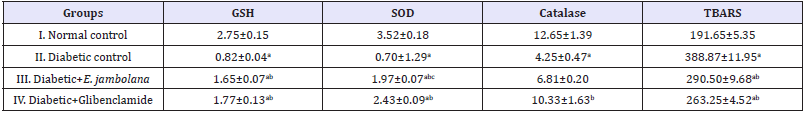
Data are expressed as mean±SEM of 6 animals in each group and significantly different (p<0.05): ain comparison to normal control; bin comparison to diabetic control group; and cin comparison to the Glibenclamide treated group. GSH: Reduced Glutathione (mg/ml); SOD: Super Oxide Dismutase (μM/min/mg protein); Catalase (μM/min/mg protein); TBARS: Thiobarbituric acid reacting substances (n mole of MDA/ml of blood)
Effect of ethanolic seed extract of E. jambolana on biomarkers of oxidative stress in tissues
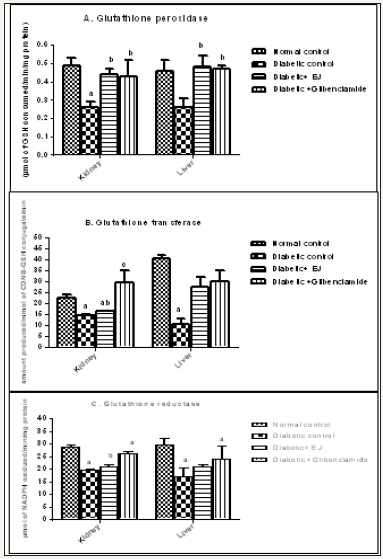
Induction of diabetes significantly reduced the activities of GPx, GST and GR in liver and kidney tissue of STZ induced diabetic animals (Group II). After 28 days treatment with ethanolic seed extract of E. jambolana (Group III) markedly increased the activity of GR, GST and GPxin comparison to the diabetic animals (Group II) which was quite similar to the effect of the drug Glibenclamide(Group IV) (Figure 1).
Figure 1: Effect of ethanolic seed extract of Eugenia jambolana on biomarkers of oxidative stress in the tissues. A. Glutathione peroxidase (GPx) activity in kidney and liver, B. Glutathione transferase (GST)activity in kidney and liver C. Glutathione reductase ( GR) activity in kidney and liver as.
Effect of ethanolic seed extract of E. jambolana on DNA damage
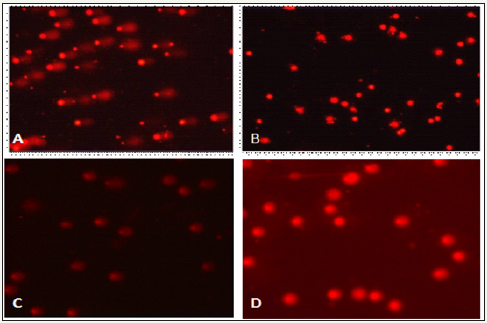
The comet assay was performed by taking fresh blood from the animals of each group in order to measure the degree of DNA damage in diabetic animals and also to measure the degree of recovery in animals of treated groups. Comets with long tails were clearly observed in case of diabetic control group animals (Group II) which indicated the DNA damage due to the oxidative stress was caused by streptozotocin (Figure 2a) whereas in normal animals (Group I) there was no such comets observed (Figure 2b). The normal control group (Group I) had circular nucleus indicating lack of DNA damage. In E. jambolana seed treated diabetic group (Group III). 2, the numbers of comets in the slide were observed with shorter tail length as compared to diabetic control group (Figure 2c). These observations clearly indicated that there is some degree of recovery to the DNA damage in the treated group animals (Figure 2d).
Figure 2: Comet test results of the DNA damage on the use of ethanolic extract of E. jambolana in diabetic rates Comet image by comet assay(A) Diabetic control rats (B) Normal control rats (C) E. jambolana seed treated rats (D) Glibenclamide treated rats.
Discussion
Many herbal extracts from indigenous and endemic Indian plants are popular and effective for their hyperglycemic, antioxidant and antihyperlepidemic activity. E. jambolana seed contains large amount of secondary metabolites notably flavanoids. The seed kernels and the seeds have been reported to possess anti-diabetic and antioxidant properties [23,20]. Based on these preliminary reports, the ethanolic seed extract of E. jambolana was used to evaluate the therapeutic potential in diabetic animals [3,47,48]. The anti-hyperglycemic activity of E. jambolana extract to significantly bring down the elevated levels of blood glucose in diabetic rats may be an essential trigger for the development of normal homeostasis during experimental diabetes and its associated complications The flavonoid rich extract of E. jambolana seed has also been reported earlier to significantly reduce mild and severe hyperglycemia in experimental animals [20,25,49-52]. Diabetes mellitus causes a disturbance in the uptake of glucose and glucose metabolism. This chronic disease results in a rise in free radical production and increase in the serum lipids in the diabetic rats. This may be due to the increased mobilization of free fatty acids from peripheral deposits [3,47]. Dyslipidemia is one of the major risk factors for the development of atherosclerosis. The increased TG and TC levels and decreased HDL-C are known risk factors for coronary heart disease (CHD). Hypertriglyceridemia is a common finding in patients with diabetes mellitus and is responsible for vascular complications [52,53]. Variety of alterations in metabolic and regulatory mechanisms, due to insulin deficiency or due to insulin resistance are responsible for the observed accumulation of lipids [48].
The present study, indicate E. jambolana extract significantly reduces the TC, TG, LDL-C and VLDL-C levels with an increase of HDL-C in treated diabetic rats. Oral administration of ethanolic extract of E. jambolanas-kernel (100mg/kg body weight), was earlier reported to alter the plasma lipoproteins (HDL, LDL, VLDLcholesterol) and fatty acid composition in STZ-induced diabetic rats and these levels were also reverted back to near normalcy by E. jambolanas-kernel or glibenclamide treatment [25,54]. Chronic hyperglycemia cause disturbances in carbohydrate, lipid and protein metabolisms together with oxidative stress are likely to affect hepatic and renal functions. Hence our study was also focused to know the protective activity of E. jambolana seed extract against hepatic and renal damage caused by diabetes. In the present study, renal functions markers viz, urea, uric acid and creatinine significantly increased in STZ induced diabetes rats but showed reversion back to near normalcy by E. jambolana seed extract or glibenclamide treatment. The hepatic enzymes such as SGOT and SGPT present in serum were used as markers in the evaluation of hepatic damage in diabetic rats. Administration of E. jambolana seed extract (250mg/kg b.w.) for 28 days significantly reduced the activities of liver marker enzymes. Histological examination of the liver section has been reported by previous workers to result in hepatic regeneration, after administration of various doses of E. jambolana Lam. These results were comparable to that of Liv.52(R) [55,56].
During diabetes, persistence hyperglycemia causes increased production of free radicals especially reactive oxygen species (ROS), attenuating antioxidant defense systems, leading to oxidative stress in variety of tissues [57,58]. Hyperglycemia increases the formation of reactive oxygen species (ROS) via several pathways, such as glucose autoxidation, the polyol pathway and non-enzymatic protein glycation [59]. All these are critically involved in the impairment of b-cell function during the development of type 2 diabetes [5].
Oxidative stress generated by STZ was found to induce the progression of glucose toxicity, triggered pancreatic β-cell dysfunction, and altered lipid metabolisms. In the present study, it was observed that there was a decreased level of SOD, catalase, and GSH and increased level of TBARS in diabetes rats. Restoration of SOD, catalase, GSH and TBARS level by the treatment of E. jambolana extract in the diabetic rats was recorded. Whereas levels of GPx, GST and GR in liver and kidney tissues decreased in diabetes rats, and after 28 days administration of E. jambolana seed extract, marked improvement in the level of GPx, GST and GR in kidney and liver tissues besides DNA tail length indicates antioxidant potentials of E. jambolana ethanolic extract. This could be attributed to flavonoids, saponins, glycosides, phenolic and triterpenoids in the extract.
Conclusion
From the present study, it can be concluded that ethanolic extract of E. jambolana seed may be useful in treating diabetes mellitus and may be used as a supplementary drug. The phytochemicals flavonoids, saponins, glycosides, and triterpenoids present ethanolic seed extract of E. jambolana, scavenge free radicals and prevent the depletion of endogenous antioxidants easing out oxidative stress. The E. jambolana extract has no hepatotocity and nephrotoxicity and was found to improve liver and kidney functions.
References
- Kesavadev J, Saboo B, Sadikot S, Das AK, Joshi S, et al. (2017) Unproven therapies for diabetes and their implications. Adv Ther 34(1): 60-77.
- American Diabetes Association (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36(Suppl 1): S67-S74.
- De Fronzo RA (2004) Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88(4): 787-835.
- Hamamdzic D, Wilensky RL (2013) Porcine models of accelerated coronary atherosclerosis: role of diabetes mellitus and hypercholesterolemia. Journal of diabetes research 2013: 1-7.
- Giugliano D, Ceriello A, PaolissoG (1996) Oxidative stress and diabetic vascular complications. Diabetes Care 19(3): 257-267.
- Chatterjee S, Khunti K, Davies MJ (2017) Type 2 diabetes. Lancet 389(10085): 2239-2251.
- Deepthi B, Sowjanya K, Lidiya B, Bhargavi RS, Babu PS (2017) A modern review of diabetes mellitus: an annihilatory metabolic disorder. J In Silico In Vitro Pharmacol 3: 1.
- Modi P (2007) Diabetes beyond insulin: review of new drugs for treatment of diabetes mellitus. Curr Drug Discov Technol 4(1): 39-47.
- Michael J, Fowler MD (2007) Diabetes treatment, part 2: Oral agents for glycemic management. Clin Diabetes 25: 131-134.
- Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414(6865): 821-827.
- Babu PA, Suneetha G, Boddepalli R, Lakshmi VV, Rani TS, et al. (2006) Database of 389 medicinal plants for diabetes. Bioinformation 1(4): 130-131.
- WHO: Footnote to Annex 3 (1980) WHO expert committee on diabetes mellitus, 2nd Report Technical Report Series 646: 1-80.
- Subash BP, Prabuseenivasan S, Ignacimuthu S (2007) Cinnamaldehyde-a potential antidiabetic agent. Phytomedicine 14(1): 15-22.
- Bhat M, Zinjarde SS, Bhargava SY, Kumar AR, Joshi BN (2011) Antidiabetic Indian plants: a good source of potent amylase inhibitors. Evid Based Complement Alternat Med 2011: 1-6.
- Mahajan S, Singh N, Subramanian SK, Chauhan P, Saxena S, et al. (2013) Diabegon, a safe and effective polyherbal therapy for type 2 diabetes mellitus. World J Transl Med 2(3): 75-82.
- Salib JY, Michael HN, Eskande EF (2013) Anti-diabetic properties of flavonoid compounds isolated from Hyphaenethebaicaepicarp on alloxan induced diabetic rats. Pharmacognosy Res 5(1): 22-29.
- Rai PK, Gupta SK, Srivastava AK, Gupta RK, Watal G (2013) A scientific validation of antihyperglycemic and antihyperlipidemic attributes of trichosanthesdioica. ISRN Pharmacol 2013: 1-7.
- Mahajan S, Chauhan P, Subramani SK, Anand A, Borole D, et al. (2015) Evaluation of “GSPF kwath”: A Gymnema sylvestre-containing polyherbal formulation for the treatment of human type 2 diabetes mellitus. European Journal of Integrative Medicine 7(3): 303-311.
- Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G (2003) Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol 85(2- 3): 201-206.
- Sharma B, Balomajumder C, Roy P (2008) Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol 46(7): 2376-2383.
- Baliga MS, Fernandes S, Thilakchand KR, D’souza P, Rao S (2013) Scientific validation of the antidiabetic effects of Syzygiumjambolanum DC (black plum), a traditional medicinal plant of India. J Altern Complement Med 19(3): 191-197.
- Modak M, Dixit P, Londhe J, Ghaskadbi S, Paul AD (2007) Indian herbs and herbal drugs used for the treatment of diabetes. Journal of Clinical Biochemistry and Nutrition 40(3): 163-173.
- Ravi K, Sivagnanam K, Subramanian S (2004) Anti-diabetic activity of Eugenia jambolana seed kernels on streptozotocin-induced diabetic rats. J Med Food 7(2): 187-191.
- Singh N, Gupta M (2007) Effects of ethanolic extract of Syzygiumcumini (Linn) seed powder on pancreatic islets of alloxan diabetic rats. Indian Journal of Experimental Biology 45(10): 861-867.
- Sharma SB, Rajpoot R, Nasir A, Prabhu KM, Murthy PS (2011) Ameliorative effect of active principle isolated from seeds of Eugenia jambolana on carbohydrate metabolism in experimental diabetes. Evid Based Complement Alternat Med 2011: 1-9.
- Arun R, Prakash MV, Abraham SK, Premkumar K (2011) Role of Syzygiumcumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J Ethnopharmacol 134(2): 329-333.
- Banerjee A, Dasgupta N, De B (2005) In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chemistry 90(4): 727-733.
- Qinna NA, Badwan AA (2015) Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des Devel Ther 9: 2515-2525.
- Trivelli LA, Ranney HM, Lai HT (1971) Hemoglobin components in patients with diabetes mellitus. N Engl J Med 284(7): 353-357.
- Stockbridge H, Hardy RI, Glueck CJ (1989) Public cholesterol screening: motivation for participation, follow-up outcome, self-knowledge, and coronary heart disease risk factor intervention. J Lab Clin Med 114(2): 142-151.
- Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28(10): 2077-2080.
- Lopes-Virella MF, Stone P, Ellis S, Colwell JA (1977) Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem 23(5): 882-884.
- Bowers LD, Wong ET (1980) Kinetic serum creatinine assays. II. A critical evaluation and review. Clin Chem 26(5): 555-561.
- Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13: 156-159.
- Fossati P, Prencipe L, Berti G (1980) Use of 3,5-dichloro-2- hydroxybenzenesulfonic acid/4-minophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26(2): 227-231.
- Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1): 56-63.
- Fuehr J (1964) Bilirubin determination in the serum according to the method of l. jendrassik, r.a.cleghorn and p. grof. Med Monatsschr 18: 183-184.
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1): 70-77.
- Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85(2): 337-341.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2): 351-358.
- Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2): 389-394.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1): 265- 275.
- Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175(1): 184-191.
- Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105: 114-121.
- Worthington DJ, Rosemeyer MA (1974) Human glutathione reductase: purification of the crystalline enzyme from erythrocytes. Eur J Biochem 48(1): 167-177.
- Habig WH, Pabst MJ, JakobyWB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22): 7130-7139.
- Silva VM, Vinagre CG, Dallan LA, Chacra AP, Maranhão RC (2014) Plasma lipids, lipoprotein metabolism and HDL lipid transfers are equally altered in metabolic syndrome and in type 2 diabetes. Lipids 49(7): 677- 684.
- Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MV, et al. (2013) Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med 13: 37.
- Keller AC, Ma J, Kavalier A, He K, Brillantes AM, et al. (2011) Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine 19(1): 32-37.
- Grover JK, Vats V, Rathi SS (2000) Anti-hyperglycemic effect of Eugenia jambolana and Tinosporacordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol 73(3): 461-470.
- Sharma SB, Tanwar RS, Nasir A, Prabhu KM (2011) Antihyperlipidemic effect of active principle isolated from seed of Eugenia jambolana on alloxan-induced diabetic rabbits. J Med Food 14(4): 353-359.
- Tanwar RS, Sharma SB, Singh UR, Prabhu KM (2011) Antiatherosclerotic potential of active principle isolated from Eugenia jambolana in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med 2011: 1-9.
- Garber AJ (2002) Attenuating CV risk factors in patients with diabetes: clinical evidence to clinical practice. Diabetes Obes Metab 4(Suppl 1): S5-S12.
- Ravi K, Rajasekaran S, Subramanian S (2005) Antihyperlipidemic effect of Eugenia jambolana seed kernel on streptozotocin-induced diabetes in rats. Food Chem Toxicol 43(9): 1433-1439.
- Sisodia SS, Bhatnagar M (2009) Hepatoprotective activity of Eugenia jambolanaLam. In carbon tetrachloride treated rats. Indian J Pharmacol 41(1): 23-27.
- Donepudi AC, Aleksunes LM, Driscoll MV, Seeram NP, Slitt AL (2012) The traditional ayurvedic medicine, Eugenia jambolana (Jamun fruit), decreases liver inflammation, injury and fibrosis during cholestasis. Liver Int 32(4): 560-573.
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865): 813-820.
- PrasathGS, Sundaram CS, Subramanian SP (2013) Fisetin averts oxidative stress in pancreatic tissues of streptozotocin-induced diabetic rats. Endocrine 44(2): 359-368.
- Mohamed J, Shing SW, Idris MH, Budin SB, Zainalabidin S (2013) The protective effect of aqueous extracts of roselle (Hibiscus sabdariffa L. UKMR-2) against red blood cell membrane oxidative stress in rats with streptozotocin-induced diabetes. Clinics (Sao Paulo) 68(10): 1358-1363.
© 2018 GBKS Prasad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






