- Submissions

Full Text
Advances in Complementary &Alternative Medicine
The Whole Truth about Coenzyme Q10 You May Not Find Elsewhere
Enno Christian Kurt Freye* and Hans Peter Strobel
Department of Galenics, University of Düsseldorf, Germany
*Corresponding author: Enno Christian Kurt Freye, MD, PhD, Energy Development, Davos/Switzerland and the University of Duesseldorf/Germany
Submission: March 26, 2018; Published: April 05, 2018

ISSN: 2637-7802Volume2 Issue2
Abstract
Objectives: Numerous CoQ10 preparations are on the market of which however, very little is known about their bioavailability and most of all there is no information regarding their efficacy on the mitochondrial electrical transport chain, being the prime driver for ATP synthesis.
Methods: Aside from own experimental results, data from other papers were taken to evaluate various other CoQ10 formulations and their ingredients regarding bioavailability and their efficacy on the synthesis of ATP formation within the mitochondria.
Results: Two Q10 formulations (Greenspeed® and Q10 Revolution®) do demonstrate a substantial increase in bioavailability, while at the same time also increase the formation of ATP within the mitochondria. Contrary, other formulations, in spite of their higher bioavailability, they however lack any kind of conclusive data demonstrating an increase in ATP formation, being a necessary constituent in the overall function of cells. Also, the touted higher efficacy of ubiquinol has never been demonstrated conclusively, and some of the ingredients within certain Q10 formulations, do contain toxic substances just to increase bioavailability however, having a toxic side effect on mitochondria.
Conclusion: So far only two CoQ10 preparations (Q10 Revolution® and Greenspeed®) on the European market have demonstrated to have a sufficiently high bioavailability while at the same time also showing a significant efficacy on cellular function in regard to ATP synthesis.
Keywords: CoQ10; Q10 formulation; Bioavailability; ATP synthesis; Greenspeed®, Q10 Revolution®
Introduction
CoQ10 by the name of ubiquinone, is a vitamin-like nutrient that plays an essential role in the energy production of all cells. It is also known as coenzyme Q10 because its chemical structure is that of a 1,4-benzoquinone, where Q refers to the quinone group while the number 10 refers to the number of isoprenoid sub-units. It is ubiquitously distributed in practically all cells, being the reason why it's called ubi-quinone, which gives fuel to the mitochondria for a sufficient function [1].
Mode of Action of CoQ10
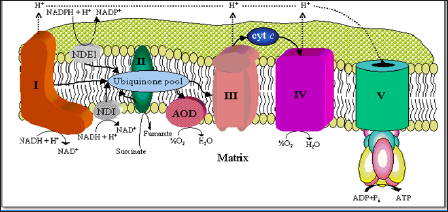
The main function of CoQ10 in the body is the production of cellular energy or adenosine triphosphate (ATP). It is the most critical component in all mitochondria (Figure 1) which are present in practically every cell in our body totaling from 6002000 mitochondria per cell [1]. The mitochondria are in fact fuel organelles where the biological energy called ATP (adenosine triphosphate) is produced. In addition, CoQ10 is also a potent antioxidant and it helps protect tissues and cellular components in the body from oxygen radicals (ROS). In addition, CoQ10 has been shown to preserve the myocardial sodium-potassium ATPase activity while stabilizing myocardial calcium-dependent channels while other important functions of CoQ10 such as cell signalling and gene expression have also been described [1,2].
CoQ10 is a crucial component within the electron transport chain (respiratory chain) in the mitochondria where energy is derived by a process called oxidative phosphorylation from the fuel products of fatty acid and where acetyl-l-carnitine acts like a shuttle, as well as protein and carbohydrate metabolism. These basic fuels are converted into biological energy called adenosine triphosphate (ATP; Figure 1) which finally drives the cellular machinery and all the biosynthetic processes such as hormonal or neurotransmitter synthesis. In this regard CoQ10 is the essential cofactor for all activities of the enzyme systems within the mitochondria affecting complex I, II, III and IV in the electron transport chain [3,4]. Here CoQ10 shifts electrons from complex I (nicotinamide adenine dinucleotide dehydrogenase or NADH) and complex II (succinate dehydrogenase) to complex III (ubiquinone-cytochrome C reductase) by virtue of its ability to either accept protons, resulting in the formation of the reduced form of ubiquinone or ubiquinol, or by virtue of oxidation releasing protons into the intermembrane space, which later are used to activate the ATPase at complex V. It is during this process of electron transfer along the electron transport chain that the vital biological energy ATP from ADP is generated (Figure 1). Because today's average diet supplies contains only a small amount of CoQ10 and it is estimated that a typical Western diet provides about 5mg CoQ10 a day [5]. Therefore, supplementation is a necessity for physically active adults, certainly in the elderly population and especially for those who have a chronic disease, since the production of CoQ10 declines with age [6] and in mostly all chronic ailments there is a low concentration of Q10 within the body. Since there are a number of Q10 preparations on the market which all claim to have superior efficacy, we undertook the aim to evaluate the prime Q10 brands on the European market in regard to their bioavailability but also in regard to their efficacy, regarding synthesis of ATP within the electrical transport chain of mitochondria.
Figure 1:The mitochondrion with its critical components the electron transport chain (ETC), where the ubiquinone pool (CoQ10) as well as NADH (or Q1) act as fuel leading into the final step, the generation of energy or ATP. Adapted from Enno Freye: Acquired Mitochondriopathy,. Springer 2012
Material and Methods
Aside from own experimental results, data from published results as found on pubmed.org, other search sites and publications being referenced by the companies, were taken to compare various CoQ10 formulations among each other. The data were screened in regard to other constituents being used to increase bioavailability of the specific product. In addition, data were also screened in regard the claimed efficacy of the product, and where the synthesis of ATP formation was considered the end-point of a sufficient activation within the electron transport chain of mitochondria.
Results
Differences in bioavailability in different CoQ10 formulations
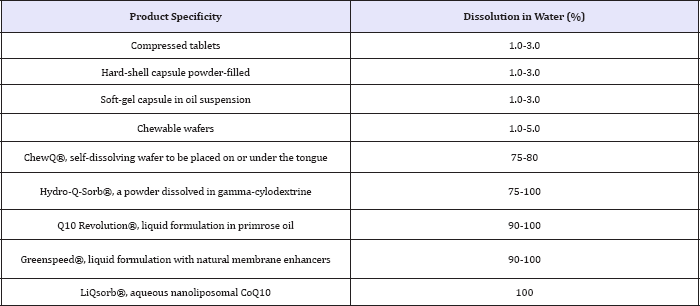
Table 1: Typical dissolution profiles of various coenzyme Q10 products. Adapted and modified from [14].
The most commonly available formulations of CoQ10 are either based on powder, in form of tablets, in form of two-piece capsules, or as soft gel capsules containing an oil suspension. This is because pure CoQ10 is insoluble in water and has only limited solubility in oils and fats. Because of the poor solubility of Q10, powder-based products show poor dissolution in aqueous media, resulting in poor absorption from the gastrointestinal tract with very low bioavailability in humans. Therefore different lipids had been added in order to increase absorption and enhance bioavailability, and these claims were based on both laboratory tests (dissolution test and cell culture studies using Caco-2 cells) as well as on plasma concentrations studies in human and animals [7-10].
In order to improve the solubilization profile of CoQ10, several preparations do stand out as having a solubilization technique which is superior to the commonly used formulations in compressed tablets, hard-shell of soft gel capsules (Table 1).
The two CoQ10 products Greenspeed® and Q10 Revolution® have demonstrated superior bioavailability when compared with other products [11,12]. In addition, the liposomal CoQ10® is a liquid preparation containing CoQ10 in a lipid and it has been shown to be superior in laboratory tests and in human bioavailability studies resulting in a higher absorption through the intestinal tract when compared to capsules [8,13]. Thus, liposomal CoQ10 purportedly represents an ideal formulation with enhanced bioavailability for patients requiring oral CoQ10 therapy such as infants, children, elderly, and those with difficulty swallowing. Another product that seemingly is suited for individuals who do not wish to or are unable to swallow tablets or capsules is ChewQ®, a chewable CoQ10 tablet formulation using the HydroQsorb® technology to ensure the highest bioavailability possible (aka plasma concentrations) by use of the additives Xylitol and gamma-cyclodextrine. Laboratory tests based on dissolution testing and cell culture studies had shown a 6 times higher CoQ10 uptake by Caco-2 cells [9]. Again, all these studies do show an increase in plasma levels (Table 1) they, however, do not reflect the amount of Q10 being taken up by cells.
While these claimed advantages regarding solubilization and absorption through the intestinal tract, which result in a marked increase in plasma Q10-concentration, however, there is a downside effect. This is because none of these studies actually do reflect the actual amount of Q10 that will get into the cell being the actual site of action. And in order to get an idea about the efficacy of a product, it is not the plasma level that is of importance. Much more the amount of Q10 that is used within the cell and which boost mitochondrial function resulting in a higher output of ATP synthesis is the most important parameter. As outlined in Table 1, the major drawback in formulations using CoQ10 capsules is their very poor dissolution, resulting in a low level of bioavailability. This is because a capsule, even in an oily suspension, cannot readily traverse through the mucous layer of the intestinal tract. Being covered by a thin aqueous film, the mucous layers of the intestinal wall are unable to let any potentially water insoluble agent pass through, and the active compound will not reach the systemic circulation. Such disadvantage can be circumvented by using a nano dispersion where practically all of CoQ10, because of their minute nano size particles resulting in anything of 300 nanometers or smaller, are able to pass through the tight junction of the intestinal cell wall, resulting in a nearly 100% absorption as demonstrated in Table 2.
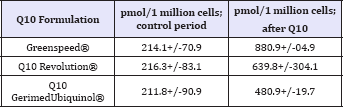
Table 2: The amount of adenosine triphosphate formation (ATP in pmol/1 million cells) within leucocytes of volunteers following 5 weeks of intakes in different CoQ10 formulations [25].
However, regular use of Q10-nanodispersion has to be reconsidered with caution. This is because previous in-vitro studies with pulmonary cells which had been exposed to industrial nanoparticles outlined a marked cytotoxic effect [14]. Because of the change in the physicochemical properties of the original molecule [15], nanoparticles practically are able to penetrate though all lipid barriers resulting in a down regulation of cellular growth followed by apoptosis, a reaction that is independent of the concentration of the nano particle being used [16]. One important finding was that the solubility of the nano particle strongly influences toxicity. As such, low concentrations of nano zinc oxide for instance, trigger a sharp decline in cell metabolism and in the proliferation of cells. At higher concentrations, however, toxicity actually drops. This may have been due to the fact that nano zinc oxide particles clump together at higher concentrations. In addition, uncoated iron oxide particles regardless of their concentration, are particularly toxic, while the coated iron oxide particles showed no side effect on cellular function [17]. And since all preparations based on a nano technology behave in a similar fashion, the safety of nano Q10- particles, for instance such as found in PureSorb Q40 TM (Nisshin Pharma) their use in humans tends to be more than questionable. This is also because new studies revealed that nanotechnology lack any kind of safety information [18]. As such the US Food and Drug Administration (FDA) currently do not specifically require safety data for foods added with nano particles. It only does require manufacturers to provide superficial tests showing that the food goods they are advertising are safe - be it beer, baby or vitamin products-and are not harmful. Up to this date, there are only a few published industries, government or scientific studies of nano particles on health and the environment. While it is known that nano particles to be more chemically reactive and more bioactive [19], such safety studies should be done with any kind of nano Q10 formulation which has a bioavailability of up to 100%, and where nano particles act as a potential toxic agents to mitochondria. As to this day, any nano Q10 preparation should not be used unless proven otherwise.
Purported advantage of ubiquinol over ubiquinone
As mentioned above, ubiquinol is the reduced version of CoQ10 (aka ubiquinone). They're actually the same molecule, but when CoQ10 is reduced it takes on two electrons, which turns it into ubiquinol. Whether CoQ10 is in the blood, the lymph or inside the mitochondria of the cells, it shifts from one form to the other depending what is needed in this particular situation. While in the blood and the lymph, CoQ10 primarily serves as an antioxidant, in the mitochondria, however, it supports the energy metabolism by switching between the two forms back and forth thousand times per second. This flipping back and forth between these two molecular forms is part of the process that transforms food into energy (Figure 1) . Ubiquinone production ramps up from early childhood until our mid-to late 20s. By the time one hits 30, it begins to decline. Young people are able to use CoQ10 supplements quite well, but older people do better with ubiquinol as it's more readily absorbed. Also, people with a genetic single nucleotide polymorphism (SNP) called NQO1 lack the enzyme required to convert CoQ10 to ubiquinol in their bodies, and they definitely need to use ubiquinol or they won't get any of the benefits. Research has shown that Hispanic and Chinese populations are especially prone to having this SNP [20,21]. Nowadays, there are genetic tests that can identify whether someone has this important enzyme in sufficient amounts or not. Inspite the claimed superiority of ubiquinol, the electron-rich form of CoQ10 that the body produces naturally, and which plays an important role in the electron transport chain of our mitochondria, facilitates the conversion of energy substrates and oxygen into the biological energy (adenosine triphosphate or ATP). This preponderance of ubiquinol, however, has not been demonstrated conclusively. In spite being touted as the "better CoQ10 formulation" resulting in higher blood plasma levels, there are no data that show if ubiquinol does indeed result in a higher energy synthesis than ubiquinone. This is because the body, as seen in Figure 1, is able to reverse both types of Q10 depending what is needed within that very moment, while switching back and forth a number of times within seconds. On the other hand, relative bioavailability of CoQ10 in its reduced form (ubiquinol), has been shown to be higher than that of CoQ10 in its oxidized form, the ubiquinone, in both animal and human studies [8,13], the high plasma level has to be questioned of being of any advantage. This is because no one so far has demonstrated that a high plasma level automatically will result in a better function of cells. It is always being taken for granted that a high plasma level of any kind of agent automatically will result in a high concentration within the cell. The contrary is true, as once a substance enters the membrane barrier it will be pushed out by a multidrug resistant protein pump (or P-glycoprotein efflux Pump) within the cell membrane [22,23] being an important mechanism for various kinds of drug resistancy [24]. Thus claiming ubiquinol in favor of ubiquinone is not validated, since all tests with ubiquinol were unable to demonstrate a superior ATP synthesis when compared to ubiquinone [25]. Claiming ubiquinol as being superior to ubiquinone is actually only a marketing ploy (!) because it is not the plasma level that automatically will result in a higher production of ATP within the mitochondria. It is of importance, how much of this CoQ10 within the plasma will actually pass the cellular membrane staying within the cell in order to be available for the machinery within the mitochondria, the electron transport chain, resulting in an enhanced production of ATP. The latter has been demonstrated conclusively in a study showing higher ATP concentrations within cellular lymphocytes with a superior generation of ATP within the mitochondria of cells by using two CoQ10 preparations, i.e. Greenspeed® and Q10 Revolution®, being more effective than a classical ubiquinol product from a German company (Table 2).
These results are in contrast to studies with other preparations of Q10 where in spite the purported rise in CoQ10 plasma concentration, the net effect on ATP synthesis within the cell is not outlined. Such studies for good reason, were never done, which might have demonstrated nil effect on the mitochondria and its synthesis of ATP. Also, with other Q10 preparations prolonged dosing will be needed. For instance, rats given doses of 200-500mg/kg/day, 1-2 months of supplementation was needed to see a significant increase in brain CoQ10 concentrations (i.e.10- 30%), underlining the notion that plasma concentrations may not adequately correlate with brain or other cell function.
Q10 preparations with toxic ingredients
A product by the name of Ubichinol Q10® from the Visanto Company in Poland not only was unable to demonstrate their alleged superiority in any kind of study; it also contains toxic adjuncts which is reflected in the E numbers of constituents being used in their formulation. This is because the company, as outlined on their label, uses:
A. Canola oil for dissolution purposes, being a trans fat and an inflammation enhancer since a large portion of canola oil used in processed food has been hardened through the hydrogenation process, which introduces levels of trans fatty acids into the final product as high as 40 percent [26,27].
B. E 407 or glycerol ester of fatty acids being used for emulsification purposes. It is a chemical extract from vegetable oil, another potential transfat and an enhancer of inflammation resulting in the degradation of mitochondria [28].
C. E 901 or so lecithin bean fat, partially hardened and another transfat affecting mitochondria. What is also troubling is the fact that soy is a product from genetically modified organism (GMOs) where the genetic material has been altered using genetic engineering techniques in order to withstand the toxic herbicide glyphosate being labeled as potentially carcinogenic [29]. It is with good cause that many countries within the European Union and the world (Japan, Australia) because of health problems, abandoned the use of GMO products.
D. E322 or soy lecithin, a chemical derivative from GMO soy, used as an emulsifier; it affects the microbiome, the healthy gut bacteria with an ensuing allergy [30].
E. E1422 is another chemically modified product by the name of acetylated distarchadipate, being a resistant starch against degradation by enzymes in the gut; it may cause abdominal bloating and pain and excessive gas (flatulence) [31,32]. In addition, resistant starches may worsen the symptoms of irritable bowel syndrome (IBS) in some individuals [33].
F. E422 or vegetable glycerol often being used in E-cigarettes another chemically extracted product for tastiness and a liquefier.
G. E 407 or carrageenan, used as a thickener which can increase inflammation leading to a greater likelihood of other diseases [34], such as inflammatory bowel disease, arthritis, tendonitis, or gallbladder inflammation.
H. E150 or sulfite caramel coloring used as an additive, where intestinal problems may occur after ingestion. It's the same substance that makes the colas brown and gives the beer their amber gold, incorporating a number of advanced glycation end products (AGE) being pro-inflammatory and pro- allergenic. But most of all, it contains the cancerous byproducts known as 2-MEI and 4-MEI where the state of California added 4-MEI to its list of chemicals known to cause cancer [35].
I. E339 iii or disodium phosphate is used to enhance food characteristics like nutritional value and is also used as an emulsifier in the Polish ubiquinol formulation. This additive cannot be considered as being healthy since it is rated as an independent predictor of cardiovascular events and mortality [36].
J. It also should be noted, that the coenzyme Q10 which is built in the liver and the CoQ10 which is taken up from our food is the oxidized ubiquinone, which at any time can be transformed into its reduced form ubiquinol within the body and within seconds. While it is commonly accepted that the elderly do not have sufficient capabilities to transform ubiquinone into ubiquinol [37], it makes the consumer believe to rather use an ubiquninol preparation; however, this does not solve the problem of a sufficient transmembrane diffusion into the cell where CoQ10 actually is needed.
In summary, all these claims regarding ubiquinol's superiority are of misleading nature, being just a marketing ploy and a stretching of scientific facts in most of the marketing claims for their ubiquinol product:
a. Calling ubiquinol the only active form of Coenzyme Q10 is in fact is not true as both forms of Coenzyme Q10 are active. Ubiquinone is active in the process of cellular energy production while ubiquinol is active in the process of antioxidant protection.
b. Saying that ubiquinol is the only one being responsible for energy production is not true, since it is ubiquinone that is the first co-factor in the process of cellular energy production. Without ubiquinone, there is no energy production. Ubiquinol is formed as a by-product of ubiquinone's role in energy production, after which the resulting ubiquinol plays a role in complex III in the inner membrane of the mitochondria where the energy substrate ATP (adenosine triphosophate) is produced (Figure 1).
c. Claiming that ubiquinol is much better absorbed than ubiquinone is not based on head-to-head comparison studies but on comparisons of studies done at different times, under differing conditions, with different participant groups, and analyzed by different laboratories. Only in one small head-to- head comparison study [38], the researchers pulled the data from one-third of the participants. The reason given was lack of compliance.
d. Stating that ubiquinol is synthesized in a multi-step process in the body is wrong insofar, as the body can synthesize its own ubiquinone in a complicated 17-step process. However, there is only one step in the conversion from the ubiquinone to ubiquinol in the body, and there is an intermediate formation of a semi-ubiquinone molecule. This conversion of ubiquinone to ubiquinol is being facilitated by the reductase enzyme, and so far it was never mentioned if the actions of this enzyme can be inhibited by harmful free radicals. Since the transformation from ubiquinone to ubiquinol is done by means of the enzyme thioredoxin reductase containing selenium, and by adding selenium to a CoQ10 preparation, such transformation can be speeded up, a reason why in the product Q10 Revolution® selenium was added to the formulation.
e. The claim that the producers of the ubiquinol products have found a way to make the ubiquinol stable is actually a hoax. Ubiquinol by virtue of its nature as an antioxidant, in reality is very unstable. It is only stable in a gelatin capsule filled with nitrogen or in a dispenser which is tightly sealed. Once it is exposed to room air or to the acid and the enzymes in the stomach, ubiquinol is rapidly converted to ubiquinone.
f. Suggesting that everyone over the age of 45 years should be taking an ubiquinol formulation is a claim which was never underscored by any kind of data. While there is an age-related decline in the ubiquinol-ubiquinone ratio being caused by the reduced ability of the body's own synthesis in part it is also being caused by the accumulating oxidative stress in older adults [39].
g. Also, since the body is constantly changing by itself from the oxidized form into the reduced form and vice versa there still could be a deficiency of a sufficient supplementation. In addition, all companies who had done safety and toxicological studies with their CoQ10 formulation were using ubiquinone and not ubiquinol, blindfolding the user in making them believe that their ubiquinol formulation has undergone similar studies, which however, is not true.
h. And lastly ubiquinol is unstable and is very reactive when getting into contact with oxygen from the air, being converted into ubiquinone. That's why ubiquinol has to be sold in tight sealed bottles which needs more technique, know how for bottling, getting more expensive than the usual vials with ubiquinone. And while ubiquinol has a creamy whitelike appearance you can readily tell once the tight sealing was damaged as now it has changed into ubiquinone having a yellowish to orange color. Such change in color will also happen once alleged tight ubiquinol capsules get into contact with oxygen rapidly changing into the yellowish oxidized form of ubiquinone.
Useful and non-useful additives in Q10 formulations
In addition, Q10 is often co-administered with a known solvent such as polysorbate 20 or 80 or other solvents in order to increase solubilization [40]. While this technique does indeed solve the problem of a reduced bioavailability of CoQ10, polysorbate, however, in cultured human epidermal cells and animal studies have shown toxicity to mitochondria with cellular destruction which is due to a disintegration of cellular membranes, ensuing apoptosis or preprogrammed cell death [41-43]. Therefore, the use of polysorbate as a solubilizing agent as found in the VESisorb® technology is simply not healthy. This technique is used in a product by the name of VESisorb® Ubiquinol-QH with an increased absorption from the gut resulting in a 308% increase in peak blood levels when compared to control [44].
On the other hand, there are two formulations, which because of their additives result in a higher functional output. One is Q10 Revolution®, which inherits four natural ingredients being vitamin E, evening primrose oil, ubiquinone Q1 or NADH, and ubiquinone Q10, with no artificial additive or solvent such as polysorbate in order to increase solubilization. It is noteworthy that the additions of NADH (or Q1) acts like an ignition key at complex I, further increasing ATP synthesis. Water solubility of Q10 Revolution® is achieved by a unique formulation developed by a company in Switzerland. Contrary to in-vitro testing [9] the putative high bioavailability of Q10 Revolution® was tested in humans. There, data being derived from patients with myocardial insufficiency demonstrate low levels of plasma Q10 before administration of Q10 Revolution®. Following intake of the liquid CoQ10 preparation the median plasma concentration which was <1.4mg/L in the control period, resulted in increase in plasma levels reaching a mean value >1,7mg/l after 1 hour (Figure 2).
However, when adding the sugar molecule D-ribose to the oroducht Q10 Revolution®, originating from Poland, On the other hand, the Greenspeed® formulation similar to Q10 Revolution® both contain the sugar molecule D-ribose. This serves as a potent adjunct. D-Ribose is a five-carbon sugar, and it's completely safe to consume even for diabetics because it has no impact to blood sugar in the sense of blood glucose activating the hormone insulin. What our bodies does with ribose, is that it gets into the cells where it is converted into the adenosine base, which goes on to have the phosphate ions attached to it to create ADP and ATP (Figure 3).
Figure 2: Q10 plasma concentrations (mg/L) in patients with myocardial insufficiency (n=10; mean±SD) before and after absorption of Q10 Revolution® through the mucous membranes within the gastrointestinal tract. Unpublished data provided by the company. Adapted from [11].
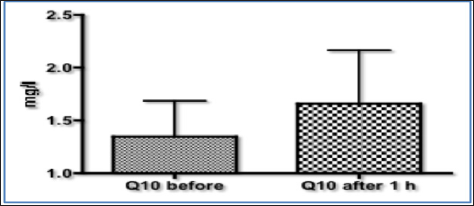
Figure 3: The constituents of adenosine diphosphate (ADP) consisting of Adenin, Ribose and two phosphorous groups, the precursor of adenosine-triphosphate (ATP), being the ultimate fuel for the cell. Incorporating the sugar D-ribose is an essential part in the formation of ATP.
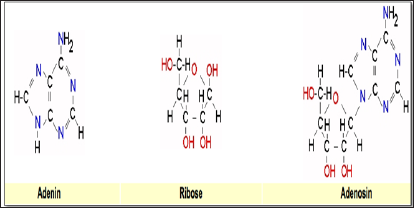
Basically, the importance of D-Ribose as a supplementation is that even though our bodies produce D-Ribose on their own, this is a very, very slow process which now is boosted up by adding D-Ribose to the formulation. D-Ribose very likely can be considered the rate limiting factor in the recovery of cardiovascular patients, or patients with myocardial insufficiency and people with chronic fatigue and even in stroke [45-47]. And while the two formulations Greenspeed® as well as Q10 Revolution® contain the necessary B-vitamins, which are also essential in the formation of the end- product adenosine triphosphate [48], the addition of D-Ribose in their formulation gives the cell an optimal source for survival. This is, because in myocardial infarction and stroke the block in blood flow causing death results in an insufficient supply of oxygen. Even though one might have death immediately within the core of the infarct, what counts is the periphery of the area of damage, where the low amount of oxygen is not enough to meet the demands of those peripheral cells. What ends up is that the cell starts to go into a lower energy state or a hibernation mode. That doesn't necessarily mean it stops having any need for energy, it still does but just at a reduced level. Once all the oxygen is used up and it still has the energy to manage, now it has this buildup of ADP without the sufficient energy to transform it into ATP (Figure 1). In order to meet the demand of ATP, it now will combine two ADPs with the help of D-ribose to create an ATP and an AMP. This ATP can now be used to supply energy, but the residual AMP or Adenosine monophosphate is something that the body now will use to form adenosine-di-phosphate, ultimately leading into the synthesis adenosine-tri-phosphate. One of the ways to get around the dilemma of formation of ATP is to supply the body with sufficient amounts of adenosine D-ribose (Figure 3), so the cells actually can produce those adenosine molecules, and have enough of those building blocks to ensure that the ATP synthase continues to run without necessarily creating free radicals. Therefore, D-Ribose is important, probably one of the most important nutritional components for a subgroup of individuals that are suffering from heart attack, stroke, and chronic fatigue as well also attenuating the so-called reperfusion injury [49].
Increase of Q10 within nerve cells results in higher neuronal activity
Aside from a sufficient bioavailability and absorption though mucous membranes of the intestinal tract, the necessity to get into the cell in order to feed the mitochondria, has been nicely shown in a separate study in healthy volunteers [12], where following intake of 25ml of Greenspeed®, the formulation induced an increase in activity of neuronal cells within the brain. Such changes are reflected in the different power spectra of the electroencephalogram (EEG).
Figure 4: Grand mean of EEG power spectra of 10 volunteers seated in a relaxed and noise-free environment in the control situation. Note the high activity within the alpha-band (7-13H7) reflecting a sedated state.
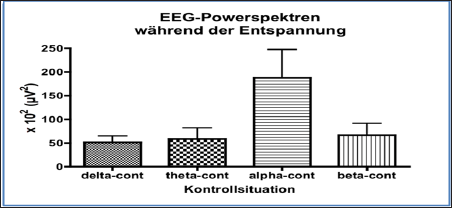
Following a run-in period of 30 minutes, EEG power spectra were taken from ten individuals seated in a relaxed position with their eyes closed. EEG potentials were picked up from Ag/AgCl stick-on electrodes attached to the scalp at position Fp1-A1 with grounding at FpZ according to the international 10/20-system [50]. EEG waves were fed into a microprocessor-controlled EEG recording machine (Lifescan®, Diatek, San Diego/USA), which performed Fast Fourier Transformation over a time epoch of 60sec yielding power spectra in the different power bands, delta (05-3Hz), theta (3-7Hz), alpha (7-13Hz) and beta (13-30Hz). Following intake of Q10 Greenspeed® and compared to the control situation (Figure 4),there is a highly significant increase of power in the fast frequency beta-domain (13-30Hz), being depicted on the right-hand side after 1 hour (Figure 5 & 6).
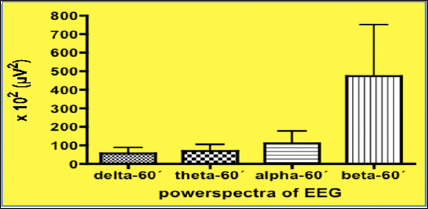
These EEG changes clearly demonstrate that after the use of Greenspeed®, beta-activity within the EEG increases by 616% (!) when compared to the control situation. Such data reflect a highly significant increase in the level of vigilance and alertness when using this preparation. Such an advantageous effect is largely related to an increase in ATP synthesis within the neuronal cells as demonstrated conclusively in leucocytes (see Table 2) using the technology of luciferase activity and which reflects the increase in ATP formation [25]. Following ingestion of Greenspeed® the present data suggest that within the allotted time frame, sufficient aliquots of CoQ10 traverse through the natural barrier, the BBB (blood brain barrier) resulting in an accumulation of sufficient amounts of CoQ10 within the central nervous system cells, which is followed by an increase in energy formation. This is mirrored in a desynchronization of EEG waves with high activity within the fast beta-band of the EEG, reflecting an increase in attention and vigilance. By using the product Greenspeed® in human volunteers and demonstrating a change within the EEG power spectra it can be concluded:
A. Sufficient amounts of CoQ10 reach the central nervous system resulting in a subsequent activation of neuronal cells.
B. The mode of action of CoQ10 at neuronal cells results in an increase of power within the fast beta domain (13-30Hz) of the EEG.
C. This increase in activity of the EEG corresponds with an increase of vigilance reaching max levels within 60 minutes. This is in marked contrast to the usual 4-5 hours with all other oral formulations.
D. Activation of central nervous activity may be of advantage in a situation of tiredness or in attention deficit when an increase of vigilance and of awareness is of advantage or even mandatory.
On the other hand, the product Q10 Revolution® which also demonstrated a significant increase in the activity of brain cells within the EEG [12], however, incorporates another important advantage. Due to its high CoQ10 content of 420mg (!) which in comparison to either 80mg or 100mg in the other preparations, this concentration is >4 fold higher. And since everything that is reabsorbed from the intestinal tract has to go through the liver where it undergoes degradation and metabolization, most of this CoQ10 by-passes the liver, resulting in higher plasma levels (Figure 2).
In conclusion, so far only two CoQ10 preparations (Q10 Revolution® and Greenspeed®) on the European market have demonstrated a sufficient high bioavailability while at the same time also showing a significant efficacy on cellular function in regard to ATP synthesis. It however is troubling to notice, how many other Q10 preparations neither have a sufficient bioavailability, are laced with additives which potentially are carcinogenic, or use solubilizing agents which by themselves are toxic to mitochondria.
Figure 5: Change of the grand mean of power within the different EEG spectra yielding a higher power within the fast frequency range, a result of Greenspeed® uptake. The shift towards the right fast spectrum suggests a higher state of attention and vigilance.
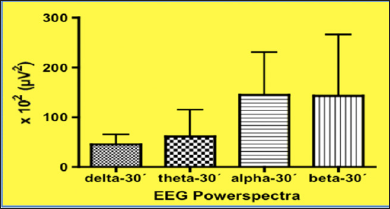
Figure 6: Further increase in EEG power spectra within the fast domain demonstrating a highly significant increase in the fast beta-range (p<0.001) when compared to the control situation, following 1 hour after intake of Greenspeed®.
Conflict of Interest
The authors declare no conflict of interest, neither working for nor holding stocks from any of the mentioned companies.
References
- Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20(6): 591-598.
- Ernster L, Dallner G (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271(1): 195-204.
- Fossilien E (2001) Mitochondrial medicine-molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci 31(1): 25-76.
- Aberg F, Appelkvist EL, Dallner G, Ernster L (1992) Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys 295(2): 230-234.
- Weber C, Bysted A, Holmer G (1997) The coenzyme Q10 content of the average Danish diet. Int J Vitam Nutr Res 67(2): 123-129.
- Chopra RK, Goldman R, Sinatra ST, Bhagavan HN (1998) Relative bioavailability of coenzyme Q10 formulations in human subjects. Int J Vit Nutr Res 68(2): 109-113.
- Miles MV, Paul H, Lili M, Peter T, Paul S, et al. (2002) Bioequivalence of coenzyme Q10 from over the counter supplements. Nutr Res 22(8): 919929.
- Molyneux S, Florkowski C, Lever M, George P (2004) The bioavailability of coenzyme Q10 supplements available in New Zealand differs markedly. N Z Med J 117(1203): 108-113.
- Craft NE (2005) Assessment of coenzyme Q10 bioavailability using a coupled in vitro digestion/Caco-2 human intestinal cell model. FASEB J Volume 19.
- Shults CW, Flint BM, Song D, Fontaine D (2004) Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson's disease. Expt Neurol 188(2): 491-494.
- Danielic R (2015) Increase in plasma concentration of Q10 following administration of a new formulation of Q10 in patients with myocardial insufficiency. JAG Group Polska, Boguszyn, Poland, p. 4.
- Freye E, Strobel HP (2016) Changes within the electroencephalogram and increase in mental concentration are related to differences in solubulisation and composition of different Q10-formulations. Nat Prod Chem Res 4: 233-238.
- Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, et al. (2007) Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4- week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol 47(1): 19-28.
- Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, et al. (2006) In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 40(14): 4374-4381.
- De Jong WH, Borm PJA (2017) Drug delivery and nanoparticles: Applications and hazards. Int J Nanomedicine 3(2): 133-149.
- Ma DD, Yang WX (2016) Engineered nanoparticles induce cell apoptosis: potential for cancer therapy. Oncotarget 7(26): 40882-40903.
- Wiesner MN, Lowry GV, Alvarez P, Dionysiou D, Biswas P (2006) Asessing the risks of manufactured nanomaterials. Environ Sci Technol 40(14): 4336-4345.
- Jelinek A, Klöcking HP (1998) In vitro toxicity of surfactants in U937 cells: Cell membrane integrity and mitochondrial function. Exp Toxic Pathol 50(4-6): 472-476.
- Pyo SM, Müller RH, Keck CM (2017) Encapsulation by nanostructured lipid carriers, in nanoencapsulation technologies for the food and nutraceutical industries. Jafari SM (Ed.), Elsevier, UK, pp. 114-135.
- Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, et al. (2000) NAD(P) H: quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 129(1- 2): 77-97.
- Kelsey KT, Ross D, Traver RD, Christiani DC, Zuo ZF, et al. (1997)Ethnic variation in the prevalence of a common NAD(P)H:quinone oxidoreductase polymorphism and its implications for anticancer chemotherapy. Br J Cancer 76(7): 852-854.
- Kartner N, Riordan JR, Ling V (1983) Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science 221(4617): 1285-1288.
- Gohel MC (2011) Novel drug delivery approaches to bypass P-Glycoprotein efflux pump-Review. Pharmainfo.net.
- Germann UA (1996) P-glycoprotein: A mediator of multidrug resistance in tumor cells. Eur J Cancer 32A(6): 927-944.
- Freye E, Strobel HP (2015) Faster recovery after exercise with phytochemicals aimed at mitochondria energy turnover-A double blind randomized placebo control study in college female soccer players. Int J Pharmacol Phytochem Ethnomed 1: 65-73.
- Sebedio JL, Christie WW (1998) Trans fatty acids in human nutrition. The Oily Press, Dundee, Scotland, UK.
- Enig MG (1995) Trans fatty acids in the food supply: A comprehensive report covering 60 years of research. (2nd edn), Silver Spring/MD, Enig Associates Inc, Maryland, USA.
- Kinyanjui T, Artz WE, Mahungu S (2003) Mono-and diglycerides of fatty acid, in Encyclopedia of Food Sciences and Nutrition. Caballero B, Trugo L, Finglas PM (Eds.), Elsevier Science, Munich, Germany, pp. 2070-2077.
- Tarazona JV, Marques CD, Tiramani M, Reich H, Pfeil R, et al. (2017) Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch Toxicol 91(8): 2723-2743.
- Gu X, Beardslee T, Zeece M, Sarath G, Markwell J (2001) Identification of IgE-binding proteins in soy lecithin. Int Arch Allergy Immunol 126(3): 218-225.
- Robertson M, Bickerton AS, Dennis AL, Vidal H, Frayn KN (2005) Insulin- sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutri 82(3): 559-567.
- Birt DF, Boylston T, Hendrich S, Jane JL, Hollis J, et al. (2013) Resistant starch: Promise for improving human health. Adv Nutr 4(6): 587-601.
- National Colloborating Centre Nursing Supp Care (2008) Irritable bowel syndrome in adults-diagnosis and management of irritable bowel syndrome in primary care. NICE Clinical Guidelines, Royal College of Nursing UK, London, UK, Volume 61.
- Tobacman JK (2001) Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ Health Perspect 109(10): 983-994.
- National Toxicology Program (2007) Toxicology and carcinogenesis studies of 4-methylimidazole (CAS NO. 822-36-6) in F344/N rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser 535: 1-274.
- Ritz E, Hahn K, Ketteler M, Kuhlmann MK, Mann J (2012) Phosphate additives in food-A health risk. Dtsch Arztebl Int 109(4): 49-55.
- Turunen M, Olsson J, Dallner G (2004) Metabolism and function of coenzyme Q10. Biochim Biophys Acta 1660(1-2): 171-199.
- Dixon B, McKinnon T, Schneider E, Brown M, Tim W, et al. (2011) Bioavailability of ubiquinone versus ubiquinol. USANA Clin Res Bull, USANA Health Sci 20: 1-3.
- Del Pozo CJ, Rodriguez BE, Navas EI, Del Pozo CB, Navas P, et al. (2014) Relationship between functional capacity and body mass index with plasma coenzyme Q10 and oxidative damage in community-dwelling elderly-people. Exp Gerontol 52: 46-54.
- Hiramaa S, Tatsuishi T, Iwase K, Nakao H, Umebayashi C, et al. (2004) Flow-cytometric analysis on adverse effects of polysorbate 80 in rat thymocytes. Toxicology 199(2-3): 137-143.
- Arechabala B, Coiffard C, Rivalland P, Coiffard LJ, DeRoeck HY (1999) Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J Appl Tox 19(3): 163-165.
- Müller RH, Rühl D, Runge S, Schulze FK, Mehnert W (1997) Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Phsrm Res 14(4): 458-462.
- Lu Y, Wang YY, Yang N, Zhang D, Zhang FY, et al. (2014) Food emulsifier polysorbate 80 increases intestinal absorption of Di-(2-Ethylhexyl) phthalate in rats. Tox Sci 139(2): 317-327.
- Liu ZX, Artmann C (2009) Relative bioavailability comparison of different coenzyme Q10 formulations with a novel delivery system. Altern Ther Health Med 15(2): 42-46.
- Mahoney JRJ (1990) Recovery of post ischemic myocardial ATP levels and hexosemonophosphate shunt activity. Med Hypotheses 31(1): 21-23
- Sawada SG, Lewis S, Kovacs R, Khouri S, Gradus PI, et al. (2009) Evaluation of the anti-ischemic effects of D-ribose during dobutamine stress echocardiography: a pilot study. Cardiovasc Ultrasound 7: 5.
- Omran H, McCarter D, St Cyr J, Lüderitz B (2004) D-ribose aids congestive heart failure patients. Exp Clin Cardiol 9(2): 117-118.
- Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ (2006) Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact 163(1-2): 94-112.
- Sato H, Ueki M, Asaga T, Chujo K, Maekawa N (2009) D-ribose attenuates ischemia/reperfusion-induced renal injury by reducing neutrophil activation in rats. Tohoku J Exp Med 218(1): 35-40.
- Jasper HH (1958) The ten twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 10: 371-375.
© 2018 Enno Christian Kurt Freye, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






