- Submissions

Full Text
Advances in Complementary &Alternative Medicine
Antidiabetic, Lipid Lowering and Oxidative Stress Reducing Potential of Piper betel Leaves Powder in Alloxan Induced Diabetic Albino Rats
ES Chauhan1 and J Aishwarya2*
Department of Food Science and Nutrition, Banasthali University, India
*Corresponding author: Jaya Aishwaya, Research scholar, Ramanujan Niwas, Banasthali Vidyapith Newai Tonk, Rajasthan, 304022, India
Submission: January 21, 2018; Published: March 22, 2018

ISSN: 2637-7802Volume2 Issue1
Abstract
Aim: Diabetes mellitus is a major issue throughout the world, every fifth person is affected with it and its associated problems. The aim of the study is to see the effect of Piper betel leaves powder in glucose and lipid levels and oxidative stress on albino rats. It can be beneficial to cure type 1 diabetes mellitus. So, that Piper betel leaves powder was taken from bench to bedside.
Method and materials: Experimental method was used to analyze the therapeutic properties of betel leaves to investigate antidiabetic, lipidlowering and oxidative-stress reducing properties on albino rats. Each group contained six albino rats. Group A was control group which was fed with normal pallet rat’s diet. Alloxan and ammonium acetate were injected and high fat high cholesterol diet were orally fed to group B,C and D every day for 45 days. Piper betel dried leaves powder (150mg/kg body weight) was mixed into the diet and fed them daily. Statistical evaluation was done using mean, standard deviation and student’s't’ test. 'P' value less than 0.05 was considered statistically significant.
Results: The results showed a significant difference at various levels in antidiabetic, lipid-lowering and oxidative-stress reducing properties as well as increased level of protein, albumin and hemoglobin in albino rats. No toxicity was found in rats and no side effects were seen.
Conclusion: Piper betel dried leaves powder 150mg/kg/body weight can be used as potential pharmaceuticals in above-mentioned diseases. Overall Piper betel leaves are beneficial for disease and its associated problems.
Keywords: Piper betel; Hemoglobin; Alloxon; Oxidative stress; Diabetes
Introduction
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both. The chronic hyperglycemia of diabetes mellitus is associated with long-term damage, dysfunction and failure of different organs particularly eyes, kidneys, nerves, heart and blood vessels. These range from autoimmune destruction of the p-cells of the pancreas with consequent insulin deficiency to abnormalities that result in resistance to insulin action. The basis of the abnormalities in carbohydrate, fat and protein metabolism in diabetes is deficient action of insulin on target tissues [1]. Coronary heart disease, stroke, atherosclerosis and hyperlipidemia are the primary cause of death. Hyperlipidemia is considered by high serum total cholesterol, low density lipoprotein, very low density lipoprotein and decline in high density lipoprotein levels. The main aim of treatment in patients with hyperlipidemia is to reduce the risk of developing ischemic heart disease or the occurrence of further cardiovascular disease or cerebrovascular disease [2,3].
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids and DNA. Chemically, oxidative stress is associated with increased production of oxidizing species or a significant decrease in the effectiveness of antioxidant barricade such as glutathione [4]. Oxidative stress is thought to be linked to certain cardiovascular disease since oxidation of LDL in the vascular endothelium is a precursor to plaque formation [5]. Iron deficiency anemia (IDA) is the most common micronutrient disorder in the world; IDA is not restricted to humans only it can affect animals also. Anemia refers to circulating shrunken red blood cells and reduced amount of hemoglobin is the most common blood disorder. Symptoms can include headaches, chest pains, and pale skin. Anemia presently influences more than 1.62 billion people globally. It is repeatedly a consequence of other diseases hampering the capacity of body to construct healthy red blood cells or breakdown of red blood cell or loss.
One of the tropical shades loving perennial evergreen creeper Piper betel commonly known as paan leaves belongs to Piperaceae family. The plant has simple, alternate, ovate, cordate, acuminate and acute leaves with male spikes which are dense and cylindrical while female spikes are pendulous [6]. It helps in curing and treatment of various diseases like halitosis, boils and abscesses, conjunctivitis, constipation, headache, hysteria, itches, mastitis, mastoiditis, leucorrhoea, otorrhoea, ringworm, swelling of gum, rheumatism, abrasion, cuts and injuries [7,8]. Piper betel possess various pharmacological properties include anti-diabetic [9,10], anti-cancer [11], anti-mutagenic [12], anti-amoebic [13], anti- giardial, anti-inflammatory [14], anti-mosquito larvicidal [15], anti-microbial [16], immunomodulatory [17], anti-ulcerogenic, radioprotective [18], anti-leishmanial [19] and anti-fungal activity [20]. Piper betel leaves are rich in moisture, protein, fats, minerals, vitamins and in many phytochemical [21]. Phytochemical such as alkaloids, phytosterol, phenols, tannins, glycosides, terpenoids and flavonoids were its main active compounds due to which these shows therapeutic effect on many diseases.
Materials and Methods
The current study was carried out in Department of Food Science and Nutrition at Banasthali University, Distt-Tonk, Rajasthan, India after approval by Institutional Animal Ethics Committee. Handling and care of animals was according to CPCSEA guidelines. Care was taken during the animal study including food, water and shelter (Housing) as prevention from infection.
Collection of plant materials
There are many varieties of Piper betel based on the color, size, taste and aroma. Some of the most popular Indian varieties are the Magadhi, Venmony, Mysore, Salem, Calcutta, Banarasi, Kauri, Ghanagete, Bagerhati, Metha and Madras. In which the variety selected is in the current study Madras easily available in our area, the leaves were purchased from the local market at Newai, Rajasthan, India.
Preparation of leaves powder
Fresh leaves of Piper betel were washed under the running tap water and then dried under the shade at room temperature. Dried leaves were powdered in electronic grinder and stored in air tight container for further use. Uses of dry leaves powder were selected so that these can easily consumed throughout the year.
Pre-supplementation phase
Animals- Twenty four male albino rats (wistar addendum strain), 4-5 weeks old and weighing 100-150gm were procured from Haryana Agricultural University, Hisar. In the pre experimental period of a fortnight, the animals were given free access to standard rat pellet diet and water.
Experimental design
After initial period of adaptation, the animals were divided into four groups in such a way that average weight of each group constituting six rats remained almost similar. Rats were housed in solid bottom plastic cages with stainless wired top. Each group was fed on different types of diet. The temperature of the animal room was maintained between 20-25 °C and weekly weight was monitored. Actual consumption of diet of every rat was determined by weighing food given as well as left over's every day.
Composition of diet
The diet for the rats comprised of standard pellets procured from Ashirwad diet, Chandigarh with the Table 1 composition.
Table 1: Control group diet.
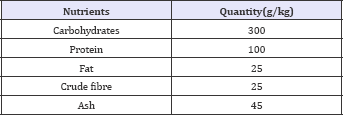
Experimental group diet
The animals in the high fat groups were administered High- Fat High-Cholesterol diet. However, both the diets were made isocaloric at the expense of carbohydrate. Casein was used as a source of protein and coconut oil and refined oil (groundnut oil) combination was used to provide fat. The mineral and vitamin mixes were prepared to provide essential and potentially beneficial mineral elements. Composition of experimental diet has been mentioned in the Table 2.
Table 2: Composition of experimental diet.

Drugs used in the study
Alloxan: Group B and D were administered with alloxan monohydrate dissolved in normal saline at a dose of 120mg/kg body weight
Ammonium acetate: Oxidative stress is known as chemical trigger free radical information and thus was used to see the effect of bioactive principle on the oxidative stress status of rats in a short period of time. Oxidative stress was induced to the C and D groups by ammonium acetate at a rate of 125mg/kg body weight.
High-fat high-cholesterol diet (HFHC): However, the diets were made isocaloric at the expense of carbohydrate. Casein was used as a source of protein and coconut oil and refined oil (groundnut oil) combination was used to provide fat. The mineral and vitamin mixes were prepared to provide essential and potentially beneficial mineral elements with water.
A. Group A: Control: Isoenergic Normal Fat Diet
B. Group B: Normal Fat Diet+Alloxan
C. Group C: High Fat and High Cholesterol diet+Ammonium acetate
D. Group D: High Fat High Cholesterol diet+Alloxan+Ammonium acetate+Piper betel leaves powder (150mg/kg body weight)
Post supplementation phase
At the end of 45 days experimental period, food was withheld overnight. The rats were anaesthetized with diethyleather the following morning blood was withdrawn from the orbital sinus into tubes. Thereafter, the animals were sacrificed by cervical dislocation. The liver, kidney, heart and brain were removed and washed with ice cold saline solution, weighed and immediately processed for biochemical analysis. Collection of blood for preparation of Serum,Lipid Extract from Liver Tissue of rats [22], Post Mitochondrial Supernatant (PMS) and Brain Homogenate were also prepared for further analysis.
Biochemical analysis of blood/serum
Estimation of haemoglobin by Sahli's method [23], glucose [24], total cholesterol [25], high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) [26], albumin [27], protein [28], triglycerides [29], uric acid [30], SGOT (Serum Glutamate Oxaloacetate Transminase) [31] and SGPT (Serum Glutamate Pyruvate Transaminase) on serum were also
Biochemical analysis on liver homogenate
Free fatty acids (FFA) [32], triglycerides (TG) [33], total cholesterol (TC) [34], phospholipids [35] were also analysed on liver tissue.
Biochemical analysis of post mitochondrial supernatant (PMS)
Estimation of Reduced Glutathione [36], Glutathione Peroxidase [37] and Superoxide dismutase [38] were determined on brain homogenate.
Statistical analysis of data
The statistical methods used for the analysis of data for the present study were: Mean, Standard deviation, T-test. If p value is <0.05 then it was considered statistically significant (s) and if not it, was considered statistically non-significant (ns).
Results
Body weight and food consumption
Table 3: Effect of HFHC diet and HFHC with Piper betel leaves powder on body weight of albino rats.
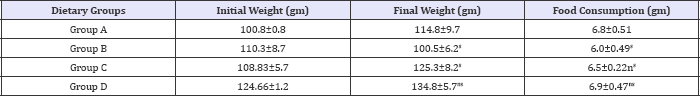
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns:
Table 4: Effect of HFHC diet and HFHC with Piper betel leaves powder on different organs weight of albino rats.
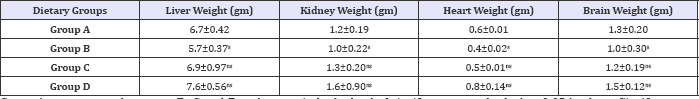
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns: Non-significant.
Body weight was decreased significantly in alloxan induced diabetic group (B) as compared to other groups (A), (C) and (D). There is no significant difference in body weight of normal group (A) and group treated by Piper betel leaves powder (D) (Table 2). HFHC diet showed significantly increased mean relative food consumption in normal fat fed rats to HFHC fed diet ones but significantly decreased in diabetic group. However, concomitant consumption of HFHC Piper betel leaves powder significantly reduced the fattening effect of high fat diet (Table 3). Research showed that increased food consumption and decrease body weight observed in diabetic control group (B) in comparison to normal group (A) indicates polyphagia condition and loss of weight due to excessive breakdown of tissue proteins. Treatment with Piper betel leaves powder group (D) food consumption improved body weight to some extent indicating control over polyphagia and muscle wasting resulted due to hyperglycaemic conditions.
Organ weight
Net increase in organ weight of animals on HFHC diet and HFHC Piper betel leaves powder diet over that of control group fed Isoenergic normal fat diet (Table 4). Liver, kidney, heart and brain weight are lower in diabetic group (B) as compared to other groups
Blood glucose status
Table 5: Effect of HFHC diet and HFHC with Piper betel leaves powder diet on blood glucose status of albino rats.
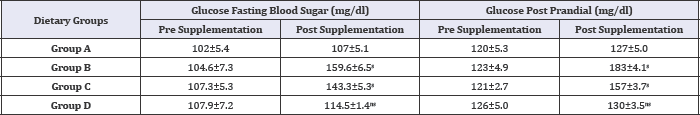
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns: Non-significant.
The effect of HFHC of Piper betel leaves powder diet group (D) on blood glucose status of albino rats has been depicted in Table 5 and Figure 1. The means blood glucose of normal fat diet diabetic rats group (B) increased significantly from rats reared on HFHC diet group (C). Simultaneous feeding of Piper betel leaves powder (D) significantly reduced the mean blood glucose level in both the groups (B) and (C). The Fasting blood glucose concentrations of diabetic rats were significantly lowered with the plant therapy. In this therapy Piper betel leaves powder fed diabetic rats showed significantly decreased blood glucose as compared to the diabetic group (B) and HFHC group (C).
Figure 1: Mean values of high-fat high-cholesterol diet and high-fat and high-cholesterol with Piper betel leaf powder diet on Blood Glucose Status of albino rats.
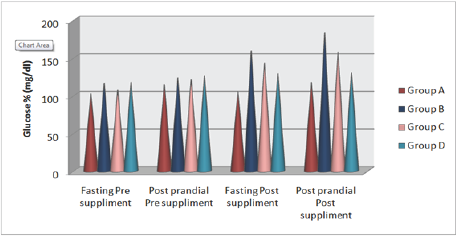
Table 6: Effect of HFHC diet and HFHC with Piper betel leaves powder diet on serum lipid profile of albino rats.
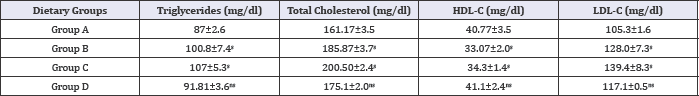
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns: Non-significant.
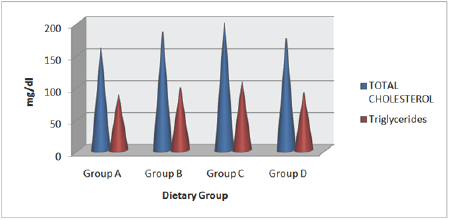
Serum lipid profile
Effects of HFHC diet and its modification by incorporation Piper betel leaves powder on serum lipid status of albino rats were evaluated. Results revealed that HFHC diets resulted in hypocholesterolemic condition in group (C) rats (Table 6 and Figure 2). Group concomitantly fed with Piper betel leaves powder (D) resulted in decline triglycerides marked decrease in total LDL-cholesterol in group (D) diabetic rats. A significant increase in cholesterol and other lipoprotein fractions. Study showed that HDL-cholesterol level was also seen in the same group. there is fall in serum concentration of cholesterol, triglycerides, and LDL-cholesterol in group (D) diabetic rats. A significant increase in HDL-cholesterol level was also seen in the same group.
Figure 2: Mean values of high-fat high-cholesterol diet and high-fat and high-cholesterol with Piper betel leaf powder diet on Serum Lipid Profile of albino rats.
Albumin, protein and haemoglobin levels
Table 7: Effect of HFHC diet and HFHC with Piper betel leaves powder diet on albumin, protein and hemoglobin levels of albino rats.
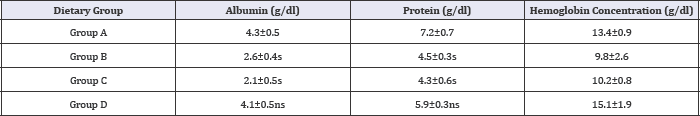
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns: Non-significant.
Table 7 showed that there is increased in serum concentration There was raised level of haemoglobin concentration noticed in of albumin and protein levels of Piper betel leaves powder fed blood parameters of Piper betel leaves powder fed diabetic rats diabetic rats i.e., group (D) then the other two groups (B) and (C). than the other three groups (A), (B) and (C).
SGPT, SGOT and uric acid levelsFigure 3: Mean values of high-fat high-cholesterol diet and high-fat and high-cholesterol with Piper betel leaf powder diet on Serum Lipid Profile of albino rats.
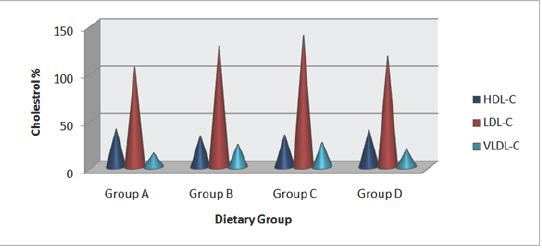
Table 8: Effect of HFHC diet and HFHC with Piper betel leaves powder diet on serum transaminease and uric acid levels of albino rats.
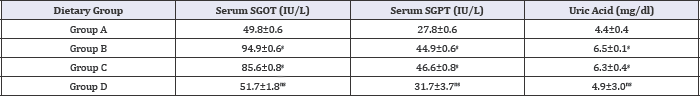
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns: Non-significant.
Table 8 and Figure 3 study showed that there is reduction in and HFHC fed group (C). A significant decrease in serum uric acid is the serum concentration of SGPT, SGOT levels in Piper betel leaves also seen between these groups. powder fed diabetic group (D) than the control diabetic group (B)and HFHC fed group (C). A significant decrease in serum uric acid is also seen between these groups.
Hepatic lipid status
Figure 4: Mean values of high-fat high-cholesterol diet and high-fat and high-cholesterol with Piper betel leaf powder diet on Hepatic Lipid Status of albino rats.

Table 9: Effect of HFHC diet and HFHC with Piper betel leaves powder diet on hepatic lipid status of albino rats.
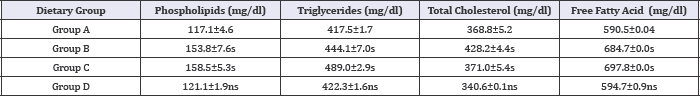
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns: Non-significant.
The effect of HFHC diet (C) and HFHC Piper betel leaves respectively. Study also showed that there is fall in concentration of powder diet (D) on the hepatic lipid status of albino rats has been Phospholipids, Triglycerides, Total Cholesterol and Free Fatty acid depicted in Table 9 and Figure 4. In contrast, group (D) showed in liver tissue of HFHC Piper betel leaves powder fed diabetic rats. a marked decrease in hepatic lipid levels to group (B) and (C)Phospholipids, Triglycerides, Total Cholesterol and Free Fatty acid in liver tissue of HFHC Piper betel leaves powder fed diabetic rats.
Oxidative stress
Table 10: Effect of HFHC diet and HFHC with Piper betel leaves powder diet on brain oxidative stress of albino rats.
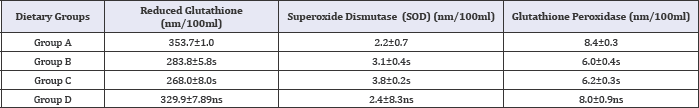
Group A was compared to group B, C and D and respectively the level of significance was checked at 0.05 levels; s: Significant, ns: Non-significant.
Blood serum and hepatic tissue examination were made to assess the oxidative stress status, which includes superoxide dismutase, reduced glutathione and glutathione peroxidase activity. As described in the Table 10 the enzyme activities were significantly increased in control diabetic group and HFHC fed group but HFHC Piper betel leaves powder supplemented with reduced the mean scores significantly. There is no significant difference in oxidative stress levels of normal group (A) and group treated by Piper betel leaves powder (D). There was marked rise in superoxide dismutase observed in controlled diabetic rats. Whereas, significant decrease in glutathione peroxidase of Piper betel leaves powder fed diabetic rats were seen. In the same way slightly decreased activity of reduced glutathione were observed in controlled diabetic rats(B) and HFHC fed rats (C) and levels were significantly regulated in Piper betel leaves powder therapy indicating modulation over brain oxidative stress.
Discussion
In the present study we focus on curative outcome of Piper betel leaves powder on alloxan induced HFHC diet fed with ammonium acetate group (D) for 45 days; to conducted biochemical analysis to establish the same then compared with the controlled diabetic group (B) and HFHC fed with ammonium acetate group (C). Piper betel dried leaves powder successfully showed the positive effects against the glucose, lipid and oxidative stress levels as we are seen in the tables and results. Supplementation of leaves powder of plant resulted in a significant correction of blood glucose level with respect to alloxan induced diabetic HFHC fed group. Oral fed of Piper betel leaves extract for 30 days to diabetic rats significantly improved the body weight. This point out the positive role of Piper betel leaves in diabetes mellitus [19,39,40]. Following the leaves powder supplementation in diabetic HFHC fed rats there was a significant recovery in hepatic lipid level also the same result found in [41]. The control levels of oxidative stress where the degree of recovery was more significant after leaves powder enhancement in the diet of group (D) due to presence of many anti-oxidants and phytochemicals its help in detoxification [40,42-44]. These recoveries were more effective when treatment of composite of leaves powder was used which primarily focuses on the antidiabetic, anti-hyperlipidemic and anti-oxidative stress activity of the plant leaves powder. In order to decide that the plant leaves powder used by us, had any toxicity in general, we measured SGOT and SGPT activities which are important signs of common toxicity. There was consideration of reduction in these parameters after separate and composite of leaves powder treatment in respect to diabetic group. This suggested that the plant leaves powder did not cause any toxicity induction and recovered the general toxicity that is noted in diabetic state. Hyperlipidemia associated with hyperglycemia, and oxidative stress leads to the amplified levels of glucose, SOD, reduced glutathione and glutathione peroxidise. After the supplementation of leaves powder the levels were near about the normal levels. This leads that Piper betel can be used in curing potential pharmaceuticals for diabetics, hyperlipidemic and oxidative stress patients and many diseases conditions.
Conclusion
Herbal plants are there by the origin of life in the earth but they are more in public interest these days. Piper betel is one of the famous climbers used for treating numerous diseases as mentioned above. Richest source of nutrients and phytochemical its all parts are used as medicine for humans and animals without any toxicity or side effects. Piper betel dried leaves powder successfully showed the affirmative effects against the glucose, lipid and oxidative stress levels on rats. This leads that Piper betel can be used in curing potential pharmaceuticals for diabetics, hyperlipidemic and oxidative stress levels as well as effect full for other parameters like hemoglobin, albumin, protein, HDL cholesterol and LDL cholesterol. It can be used in different form like incorporating in our food products; table or capsule form as well as we can take the form of powder with normal water.
Acknowledgment
The authors are thankful to the Banasthali Vidhyapith, Rajasthan, India and the department of Food Science and Nutrition for providing us lab facilities and financial assistance.
Conflict of Interest
The authors state that there are no conflicts of interest.
References
- Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94(3): 311-321.
- Sudha SS, Karthic R, Naveen, Rengaramanujam J (2011) Anti hyperlipidemic activity of Spirulina platensis in Triton x-100 induced hyperlipidemic rats. Hygeia J D Med 3(2): 32-37.
- Gunjan M, Ravindran M, Jana GK (2011) A review on some potential traditional phytomedicine with antidiabetic properties. International Journal of Phytomedicine 3(4): 448-458.
- Yoshikawa T, Naito Y (2002) What is oxidative stress? JMAJ 45(7): 271276.
- Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35(5): 1147-1150.
- Saravanan R, Prasad NR, Pugalendi KV (2003) Effect of piper betel leaf extract on alcoholic toxicity in the rat brain. J Med Food 6(3): 261-265.
- Warrier PK, Nambair VPK, Ramankutty C (1995) Indian medicinal plants: A compendium of 500 species. Arya Vaidya Sala, Kottakal, Orient Longman, Kerala, India.
- Shukla R, Sachan S, Mishra A, Kumar S (2015) A scientific review on commonly chewing plants of Asians: Piper betel Linn. Journal of Harmonized Research in Pharmacy 4(1): 1-10.
- Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87(1): 4-14.
- American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1): S62-S69.
- Guha P (2006) Betel leaf: The neglected green gold of India. Journal of Human Ecology 19(2): 87-93.
- Rai MP, Thilakchand KR, Palatty PL, Rao P, Rao S, et al. (2011) Piper betel Linn (betel vine), the maligned Southeast Asian medicinal plant possesses cancer preventive effects: time to reconsider the wronged opinion. Asian Pac J Cancer Prev 12(9): 2149-2156.
- Kanjwani DG, Marathe TP, Chiplunkar SV, Sathaye SS (2008) Evaluation of immunomodulatory activity of methanolic extract of Piper betel. Scand J Immunol 67(6): 589-593.
- Nair R, Chanda S (2008) Antimicrobial activity of terminalia catappa, manilkara zapota and piper betel leaf extract. Indian J Pharm Sci 70(3): 390-393.
- Chang MC, Uang BJ, Tsai CY, Wu HL, Lin BR, et al. (2007) Hydroxychavicol, a novel betel leaf component, inhibits platelet aggregation by suppression of cyclooxygenase, thromboxane production and calcium mobilization. Br J Pharmacol 152(1): 73-82.
- Bhattacharya S, Banerjee D, Bauri AK, Chattopadhyay S, Bandyopadhyay SK (2007) Healing property of the Piper betel phenol, allylpyrocatechol against indomethacin-induced stomach ulceration and mechanism of action. World J Gastroenterol 13(27): 3705-3713.
- Kumarasinghe SP, Karunaweera ND, Ihalamulla RL, Arambewela LS, Dissanayake RD (2002) Larvicidal effects of mineral turpentine, low aromatic white spirits, aqueous extracts of Cassia alata, and aqueous extracts, ethanolic extracts and essential oil of betel leaf (Piper betel) on Chrysomya megacephala. Int J Dermatol 41(12): 877-880.
- Bhattacharya S, Subramanian M, Bauri A, Kamat JP (2005) Radioprotecting property of the ethonolic extract of the Piper betel leaf. J Radiat Res 46(2): 165-171.
- Radhika K, Kumaravel B, Thamizhiniyan V, Subramanian S (2013) Biochemical evaluation of antidiabetic activity of Piper betel leaves extract in alloxan-induced diabetic rats. Asian J Research Chem 6(1): 76-82.
- Aishwarya J, Chauhan ES, Singh A, Tiwari A (2016) A review: Nutraceuticals properties of piper betel (Paan). American Journal of Phytomedicine and Clinical Therapeutics 4(2): 28-41.
- Aishwarya J, Chauhan ES (2016) Proximate and phytochemical scrutiny of piper betel leaves powder. International Journal of Ayurveda and Pharmaceutical Chemistry 5(2): 197-204.
- Folch J, Lees M, Sloane SGH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1): 497-509.
- Sharma S (2007) Experimental and techniques in biochemistry. (1st edn), Galgotia publication pvt ltd, New Delhi, India.
- Trinder P (1969) Ann Clin Biochem 6: 24.
- Grove TH (1997) Clin Chem 25: 560.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6): 499-502.
- Webster D (1977) Clin Chem 23: 663.
- Peters T (1968) Determination of total proteins. Clinical Chemistry 14: 1147-1159.
- McGowan MW (1983) Clin Chem 28: 538.
- Caraway WT (1955) Determination of uric acid in serum by a carbonate method. Am J Clin Pathol 25(7): 840-845.
- Reitman S, Frankel S (1957) J Clin Inevest 34: 131.
- Lowry RR, Tinsley IJ (1976) Rapid colorimetric determination of free fatty acids. J Am Oil Chem Soc 53(7): 470-472.
- Parekh AC, Jung DH (1990) Cholesterol determination with ferric acetate-uranium acetate and sulphuric acid-ferrous sulphate reagents. Analytical Chemistry 42(12): 1423-1427.
- Rice EW (1970) Triglycerides in serum. In: Roedrick P, McDonal RP (Eds.), Standard Methods in Clinical Chemistry, Academic Press, New York, USA, p. 215.
- Chen PS, Toribara TY, Warner H (1956) Anal Chem 28: 1756.
- Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25(1): 192-205.
- Necheles TF, Boles TA, Allen DM (1968) Erythrocyte glutathione- peroxidase deficiency and hemolytic disease of the newborn infant. J Pediat 72: 319-324.
- Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3): 469-474.
- Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD (2005) Antidiabetic activities of aqueous and ethanolic extracts of piper betel leaves in rats. J Ethnopharmacol 102(2): 239-245.
- Santhakumari P, Prakasam A, Pugalendi KV (2003) Modulation of oxidative stress parameters by treatment with piper betel leaf in streptozotocin induced diabetic rats. Indian Journal of Pharmacology 35(6): 373-378.
- Venkadeswaran K, Muralidharan AR, Annadurai T, Vasanthakumar, Ruban V, et al. (2014) Antihyper cholesterolemic and antioxidative potential of an extract of the plant, piper betle, and its active constituent, eugenol, in triton wr-1339-induced hypercholesterolemia in experimental rats. Evidence-Based Complementary and Alternative Medicine Vol. 2014.
- Verma S, Gupta ML, Dutta A, Sankhwar S, Shukla SK, et al. (2010) Modulation of ionizing radiation induced oxidative imbalance by semifractionated extract of Piper betel: an in vitro and in vivo assessment. Oxid Med Cell Longev 3(1): 44-52.
- Vyawahare NS, Bodhankar SL (2007) Neuropharmacological profile of Piper betel leaves extract in mice. Pharmacologyonline 2: 146-162.
- Chakraborty D, Shah B (2011) Antimicrobial, antioxidative and antihemolytic activity of piper betel leaf extracts. Int J Pharm Pharm Sci 3(3): 192-199.
© 2018 ES Chauhan, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






