- Submissions

Full Text
Advances in Complementary &Alternative Medicine
Complementary Treatment of Mood Disturbances in Terminally Ill Oncological Patients with the Aphanizomenon Flos Aquae Extract Klamin®
Bellingeri Paolo1, Bonucci Massimo2 and Scoglio Stefano3*
1AUSL Alessandria, Italy
2Casa di Cura San Feliciano, Italy
3Nutritheraphy Research Center, Italy
*Corresponding author: Stefano Scoglio, Centro di Ricerche Nutriterapiche, via I maggetti 14, 61029 Urbino, Italy
Submission: January 02, 2018; Published: February 22, 2018

ISSN: 2637-7802Volume1 Issue3
Abstract
Klamin® is a proprietary extract of the microalgae Aphanizomenon flos aquae, which concentrates both the phenylethylamine (PEA) naturally contained in the microalgae, and microalgal molecules, such as AFA-phycocyanins and mycosporine-like amino acids (MAAs), which act as physiological MAO-B inhibtors, thus allowing PEA to reach the brain and explete its numerous neuro-modulating activities. Based on this premise, and on previous studies showing the mood enhancing activity of the extract, we tested its ability to improve the very difficult condition of terminal cancer patients, with special reference to their levels of depression, anxiety and fatigue. The results obtained were positive, in spite of the extreme condition of the patients, with significant improvement of their levels of depression and anxiety, and a lesser but still relevant improvement of their level of fatigue. This preliminary study warrants the need for further research on this very relevant subject.
Introduction
In these last few years, there has been a considerable rise in the development of palliative cures for neoplastic patients in a state of terminal illness [1]. In addition to controlling pain, which certainly remains the primary objective, relief has also been sought for such other aspects as fatigue, asthenia, depression and anxiety frequently associated as non secondary symptoms [2,3]. The use of allopathic drugs to cure these symptoms may not be the most opportune solution, as the terminal patients prefer non invasive palliative methods [4]. They in fact increase the risk of creating further discomfort to patients who are already forced to undergo aggressive drug therapies, and of increasing negative side effects created by the frequent anti-tumoral medication used by the patients [5,6].
The research was thus oriented to also search for approaches that we can define as complementary [7], different from classic pharmacologic treatments, and possibly founded upon nutritional [8,9] and/or natural factors [10,11]. A research has been developing into treatments which aimed to limit symptoms without presenting at the same time excessive side effects. For example, an appropriate diet can be a very useful tool in terms of treatment. All data gathered in international medical literature stress the possibility that nutrition is capable of improving the quality of life, especially in terminally ill oncological patients characterized by: difficulty in eating; glossitis; significant alterations in taste or notable intestinal hypomotility, with constipation resistant to the most common treatments. Many of these problems are treatable at a dietary level [12].
More problematic, however, is using only ordinary nutritional treatments when confronting psychological and neurological states which characterize the advanced oncological patient, such as fatigue, asthenia, depression and anxiety. Always in the context of nutraceutical and natural substances, and on the base of medical-scientific literature of the last few years, we verified that the neuro-modulating, dopaminergic, and neuro-protective properties of the specific extract Klamin®, from Klamath microalgae (Aphanizomenon flos aquae), also characterized further by the absence of side effects, could in fact function usefully in this area, and we thus decided to initiate a preliminary experiment on 18 terminal cancer patients.
Klamin®
Klamin® provides, in only 2 tablets per day, approximately 16mg of phenylethylamine (PEA), an endogenous dopaminergic neurotransmitter, which in the algae is strengthened by the presence of further specific molecules that act as selective physiological inhibitors of MAO-B enzymes, thus allowing the ingested PEA to be protected from enzymatic inhibition and thus to reach the brain. Phenylethylamine is an endogenous amine which has been defined as the 'molecule of love', being linked to affective behavior [13], and is considered a general neuromodulator [14,15], besides acting also as an autonomous neurotransmitter [16]. The most studied role of PEA relates to its ability to activate dopaminergic transmission, which it does both by stimulating the brain nigrostriatal tissue to produce more dopamine [17] and acetylcholine [18], as well as by acting as a reuptake inhibitor of dopamine itself, with the consequent increase of the dopaminergic cascade, leading to higher production of noradrenaline, and thus to a possible improvement of energy levels and fatigue [19,20]. However, being a neuromodulator rather than a stimulant, PEA can also inhibit neurotransmitting activity if needed [21], and can also stimulate serotonine and the production of endorphins.
Depressed patients can be characterized by low levels of phenylacetic acid, a metabolite of phenylethylamine, in the urine, thus signifying low levels of PEA in the organism in general, and in the brain in particular [22]. The administration of PEA, together with a MAO-B inhibitor, has been able to improve the condition of depressed patients, without side effects and without inducing drug tolerance [23]; while Klamin® itself has already proven its ability to counteract both depression and anxiety in gynecological patients [24,25].
Being a small molecule, PEA passes easily through various physiological barriers, including the haemato-encephalic barrier; but if ingested by itself, PEA is rapidly destroyed, already at the gastro-intestinal level, by monoamine oxidase B (MAO-B) enzymes. The Klamin® extract naturally contains molecules endowed with a very significant MAO-B selective inhibition activity, namely AFA- phycocyanins (a unique type of phycocyanins present in Klamath algae); the mycosporine-like amino acids (MAAs) porphyra and shinorine; and a unique molecule called AFA-phytochrome. Their inhibition of the MAO-B enzyme is reversible, and thus more physiological than the activity performed by synthetic molecules, such as seligiline, with their irreversible destruction of the enzyme. This could explain Klamin®'s lack of any neurological side effects [26].
Thanks to its content of AFA-phycocyanins, as well as an array of other anti-oxidant substances (such as polyphenols and MAAs), Klamin® can also perform a significant neuroprotective activity. Animal studies on C-phycocyanin, a component of the AFA-phycocyanin's phycobilisome, have already shown its neuroprotective ability [27]; while a preliminary test on the specific AFA-phycocyanins has shown a significant protection of neuronal cultures from different types of oxidative insult already at the nanomolar level [28]. Finally, no reports of any adverse effects caused by the Klamath algae extract have been reported, in the medical-scientific literature. This is also why we have decided to test the effects of Klamin® on the depression, anxiety and fatigue in advanced cancer patients, who are, as is to be expected, inevitably affected by such conditions.
Patients and Treatments
Eighteen patients were enrolled at random. Only patients were included who were not taking, or had not recently taken, antidepressants and anxiolytics; who were considered terminally ill, and thus were no longer subjected to pharmacological treatments such as chemotherapy, immune-modulators drugs or radiotherapy. Inclusion criteria covered only patients who were exclusively undergoing symptomatic therapies such as diuretics, cortisone, opiates and, in two cases, haloperidol. Finally, only elderly people above 65 years of age were enrolled, and both the patient's advanced age and of the advanced stage of the illness advised against adopting classical oncological treatments, such as parenteral nutrition or blood transfusions. All patients consumed two tablets a day (before breakfast and lunch) of Klamin® (1gram in total) for a period of two months.
Elderly patients, in the advanced stages of a terminal disease, pose also problems of mood disorders and depression, which are normally addressed with common tricyclic anti-depressants, or derivatives of sertraline. We wanted to see if the use of Klamin® could improve the patients's condition enough to avoid or reduce further pharmacological interventions, whose effectiveness could not be seen but in the long term (with patients who, given their condition, may not have the availability of long terms), and whose side effects would have further diminished the patient's quality of life. For an evaluation of the results the VAS scale (0-10) was used, in which 0 represents the presence of little or no mood disturbances and 10 a maximum level of mood disturbances.
Results
Comparing the symptomatology before and after treatment, there has been a significant improvement in the group of advanced cancer patients relative to their depression, anxiety and fatigue levels. In Table 1 below, the results from each single patient were reported in relation to the three areas of anxiety, fatigue and depression (mood). The statistical evaluation of the results in each area produces the following Table 2-4 and Figure 1-3.
Figure 1: Levels of Anxiety in response to the administration of Klamin®.
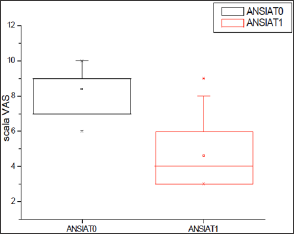
Figure 2: Levels of Fatigue in response to the administration of Klamin®.
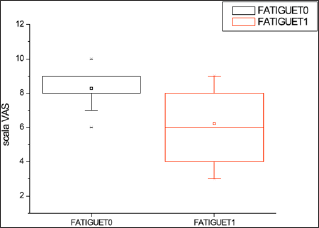
Figure 3: Levels of depression (Umore) in response to the administration of Klamin®.
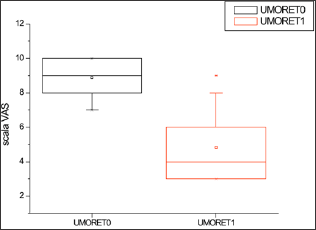
Table 1: Anxiety, Fatigue and Depression levels in response to the administration of Klamin®.

VAS scale: 0-no disturbance; 10-maximum disturbance.
Evaluation carried out at the end of 2 months of treatment.
T0=before Klamin®; T1=after 2 months of treatment with Klamin®
Table 2:
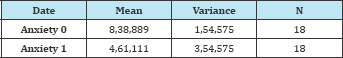
t=8,7445; p=1,06461E-7
At the 0,0.5 level, the two means are significantly different.
Table 3:
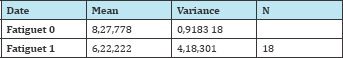
t=4,94174; p=1,23806E-4
At the 0,0.5 level, the two means are significantly different.
Table 4:
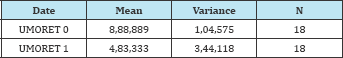
t=11,36811; p=2,29146E-9
At the 0, 0.5 level, the two means are significantly different.
Discussion
In the majority of cases the improvement has been very significant, with an average above 50% in the VAS scale, which resulted, when considering all patients, on an average 45% reduction. No significant side effects have been reported. In three cases there has been some nausea during the first three days (but the patients where afflicted by intestinal cancers). However, even these patients have completed the 60 days treatment cycle. The present study's goal was to evaluate a natural nutraceutical product, which had already proven a significant activity at the neurological level, for its ability to help alleviate some fundamental and common psychological disturbances of terminal cancer patients, thus improving the quality of their remaining life. The results have been appreciated by the patients themselves, especially in the area of depression and anxiety. It is also important to underline the high level of compliance of the enrolled patients, who were happy to receive a natural product devoid of side effects to alleviate their conditions; and also the complete lack of side effects, which explains how all the patients completed the 60 days of treatment. Based on these results, we believe it would be important to plan more specific, randomized, double blind, placebo controlled clinical studies on the use of the Klamin® extract on a larger number of terminally ill patients.
References
- Choi JY, Chang YJ, Song HY, Jho HJ, Lee MK (2013) Factors that affect quality of dying and death in terminal cancer patients on inpatient palliative care units: perspectives of bereaved family caregivers. J Pain Symptom Manage 45(4): 735-745.
- Alexander K, Goldberg J, Grodzicki KB (2016) Palliative care and symptom management in older patients with cancer. Clin Geriatr Med 32(1): 45-62.
- Kolva E, Rosenfeld B, Pessin H, Breitbart W, Brescia R (2011) Anxiety in terminally ill cancer patients. J Pain Symptom Manage 42(5): 691-701.
- Kwon SH, Im SH, Cho KW, Cho E, Yoon SJ, et al. (2012) Most advance directives written by patients with advanced cancer or their proxies request only minimally invasive treatments during end-of-life care. Am J Hosp Palliat Care 29(8): 622-626.
- Wright AA, Nancy LK, Holly GP (2014) Associations between palliative chemotherapy and adult cancer patients' end of life care and place of death: prospective cohort study. BMJ 348: g1219.
- Wu CC, Hsu TW, Chang CM, Lee CH, Huang CY, et al. (2016) Palliative chemotherapy affects aggressiveness of end-of-life care. Oncologist 21(6): 771-777.
- Vitetta L, Sali A (2006) Complementary medicine in palliative care. Aust Fam Physician 35(10): 783.
- Gillespie L, Raftery AM (2014) Nutrition in palliative and end-of-life care. Br J Community Nurs Suppl: S15-S20.
- Imoberdorf R (1997) Immuno-nutrition: designer diets in cancer. Support Care Cancer 5(5): 381-386.
- Wu TH, Chiu TY, Tsai JS, Chen CY, Chen LC, et al. (2008) Effectiveness of taiwanese traditional herbal diet for pain management in terminal cancer patients. Asia Pac J Clin Nutr 17(1): 17-22.
- Park JH, Jeon HJ, Kang HJ, Jeong IS, Cho CK (2015) Cancer-related fatigue in patients with advanced cancer treated with autonomic nerve pharmacopuncture. J Acupunct Meridian Stud 8(3): 142-146.
- Wade EV, Jain R (1984) Nutritional support: enhancing the quality of life of the terminally ill patient with cancer. J Am Diet Assoc 84(9): 10441045.
- Sabelli HC, Javaid IJ (1995) Phenylethylamine modulation of affect: therapeutic and diagnostic implications. J Neuropsychiatry Clin Neurosci 7(1): 6-14.
- Paterson IA, Juorio AV, Boulton AA (1990) 2-Phenylethylamine: a modulator of catecholamine transmission in the mammalian central nervous system? J Neurochem 55(6): 1827-1837.
- Irsfeld M, Spadafore M, Prüß BM (2013) Beta-phenylethylamine, a small molecule with a large impact. Webmedcentral 4(9).
- Federici M, Geracitano R, Tozzi A, Longone P, Di Angelantonio S, et al. (2005) Trace amines depress GABA B response in dopaminergic neurons by inhibiting G-betagamma-gated inwardly rectifying potassium channels. Mol Pharmacol 67(4): 1283-1290.
- Barroso N, Rodriguez M (1996) Action of ß-phenylethylamine and related amines on nigrostriatal dopamine neurotransmission. Eur J Pharmacol 297(3): 195-203.
- Ispida K, Mikio M, Masatoshi K, Iku U, Keiko H, et al. (2005) β-phenylethylamine stimulates striatal acetylcoline release through activation of the AMPA glutamatergic pathway. Biol Pharm Bull 28(9): 1626-1629.
- Janssen PAJ, Leysen JE, Megens AA, Awouters FH (1999) Does phenylethylamine act as an endogenous amphetamine in some patients? Int J Neuropsychopharmacol 2(3): 229-240.
- Raitieri M, Carmine DR, Bertollini A, Levi G (1977) Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol 41(2): 133-143.
- Benedetti Y (2012) Neuromodulatory and neuroprotective activity of the micro-algae Aphanizomenon Flos Aquae. University of Urbino, Faculty of Science, Italy
- Sabelli HC, Fawcett J, Gusovsky F, Javaid JI, Wynn P, et al. (1986) Clinical studies on the phenylethylamine hypothesis of affective disorder: urine and blood phenylacetic acid and phenylalanine dietary supplements. J Clin Psychiatry 47(2): 66-70.
- Sabelli H, Fink P, Fawcett J, Tom C (1996) Sustained antidepressant effect of PEA replacement. J Neuropsychiatry Clin Neurosci 8(2): 168-171.
- Scoglio S, Benedetti S, Canino C, Santagni S, Rattighieri E, et al. (2009) Effect of a 2-month treatment with klamin, a klamath algae extract, on the general well-being, antioxidant profile and oxidative status of postmenopausal women. Gynecol Endocrinol 25(4): 235-240.
- Genazzani AD, Chierchia E, Lanzoni C, Santagni S, Veltri F, et al. (2010) Effects of klamath algae extract on psychological disorders and depression in menopausal women: a pilot study. Minerva Ginecol 62(5):381-388.
- Scoglio S, Canestrari, Benedetti, Delgado E (2007a) Aphanizomenon flos aquae preparations, extracts and purified components thereof for the treatment of neurological, neurodegenerative and mood disorders.
- Rimbau V, Camins A, Romay C, González R, Pallàs M (1999) Protective effects of C-phycocyanin against kainic acid-induced neuronal damage in rat hippocampus. Neurosci Lett 276(2): 75-78.
- Scoglio S, Canestrari, Benedetti, Zolla (2007b) Extract of Aphanizomenon flos aquae and nutritional, cosmetic and pharmaceutical compositions containing the same.
© 2018 Bellingeri Paolo, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






