- Submissions

Full Text
Advances in Complementary & Alternative Medicine
Evaluation of the Antioxidant Activities of Organic Extracts from Ammodaucus leucotrichus Coss & Dur Fruit Part Harvested from the Algerian Sahara
Imad Abdelhamid El Haci1,2*, Wissame Mazari3, Mohamed Gherib4 and Fawzia Atik Bekkara1
1Department of Biology, Abou Bekr Belkaid Tlemcen University, Algeria
2Research Center on Physical and Chemical Analysis (CRAPC) Tipaza, Algeria
3Department of Biology, Abou Bekr Belkaid Tlemcen University, Algeria
4Department of Nature and Life Science, Salhi Ahmed University Center Naama, Algeria
*Corresponding author: Imad Abdelhamid El Haci, PhD, Scientific and Technical Research Center for Physical Chemical Analysis (CRAPC), BP 384, Zone Industrielle Bou-Ismail RP 42004, Algeria
Submission: December 04, 2017; Published: January 09, 2018

ISSN: 2637-7802Volume1 Issue1
Abstract
Aromatic and medicinal plants are a good source of natural preparations containing effective bioactive compounds which can be used for different applications. This work aims to evaluate the antioxidant activity of some organic extracts of Ammodaucus leucotrichus Coss & Dur fruit part. The whole plant was collected from the region of Beni Abbas (Bechar-Algeria). Five organic extracts were obtained and the evaluation of the antioxidant activity was performed by six conventional methods. Polar organic extracts exhibited more antioxidant power then non polar extracts. The level of phenolic compounds was moderate in all extracts. The investigation of the antioxidant activity of organic extracts from fruit part of Ammodaucus leucotrichus revealed a moderate activity tested by six conventional methods.
Keywords: Ammodaucus leucotrichus; Medicinal plant; Antioxidant activity; Organic extracts; Phenolic compounds
Introduction
Ammodaucus leucotrichus Coss & Dur is a medicinal plant used in folk medicine in Algeria. Ammodaucus leucotrichus is a small annual plant, 10-12cm high, glabrous with erect, finely striated stems. The leaves are finely dissected and slightly fleshy. The flowers are small, with 5 free petals. The fruit is a diachene, 6-10mm, long and is covered with dense silky white hairs. It usually flowers in early spring (February to April). The plant is common in the Algerian Sahara (Bechar, Djanet, and El Golea), its presence is also observed in the Canary Islands [1-3]. Ammodaucus leucotrichus is used for the treatment of many diseases (Table 1). In the continuity of our works on this species, the present study reports the determination of the phenolic and flavonoid contents and, also, the assessment of the antioxidant activity of different organic extracts of the fruit part of this plant [4].
Table 1: Different uses of Ammodaucus leucotrichus (Coss & Dur) in traditional folk medicine in Algeria.

Material and Methods
Plant materials
The whole plant was collected from the province of Beni- Abes (west-southern of Algeria-region of Bechar). The fruits of Ammodaucus leucotrichus were dried away from direct sunlight. Dried plant material was then crushed into a mortar and stored at very low temperature until further use [5].
Sample preparation
A powder (10g) of the fruit part of Ammodaucus leucotrichus was extracted by 100mL of different organic solvents with increasing polarity, under reflux for 3h. The extracts were then filtered and concentrated under reduced pressure at 60 °C using a rotary evaporator. The obtained residue was recovered in methanol and stored at +4 °C.
Total phenolic contents
Total phenolic was estimated by the Folin-Ciocalteu method [6]. 0.1mL of each sample was mixed with 2mL of sodium carbonate (2%) freshly prepared, the whole was mixed. After 5min, 100μL of Folin-Ciocalteu reagent (1N) were added to the mixture and left for 30min. The values of absorbance were recovered at 750nm against a blank. A calibration curve was performed under the same operating conditions using gallic acid. The results were expressed as mg gallic acid equivalent per gram of dry extract (mg GAE/g).
Total flavonoid contents
The total flavonoid content was determined by the method described by Ardestani & Yazdanparast [7]. Each sample (500μL) was mixed with 2mL of distilled water and subsequently with 150μL of a NaNO2 solution (15%). 150μL of aluminum chloride (AlCl3) solution (10%) was added and allowed to stand for 6min. Then, 2mL of NaOH solution (4%) was added to the mixture. Immediately, distilled water was added to bring the final volume to 5mL and the mixture was thoroughly mixed and allowed to stand for 15min. Absorbance of the mixture was determined at 510nm. Results were expressed as catech in equivalent per gram of dry extract (mg CEQ/g).
Study of the Antioxidant Activity
Evaluation of the total antioxidant capacity (TAC)
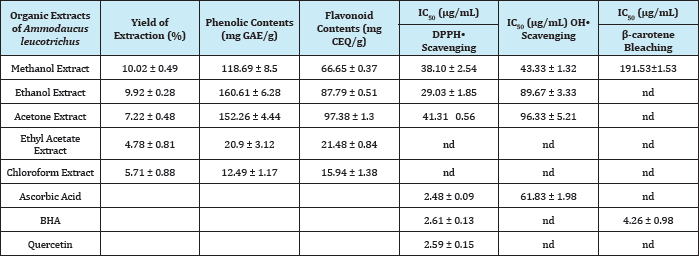
An aliquot of 0.1mL of sample solution was combined with 1ml of reagent solution (0.6M sulfuric acid, 28mM sodium phosphate, and 4mM ammonium molybdate). The tubes were capped and incubated at 95 °C for 90min. After the samples had cooled to room temperature, the absorbance was measured at 695nm. A typical blank solution contained 1mL of reagent solution and the appropriate volume of the same solvent used for the sample and it was incubated under the same conditions as the rest of the samples. For positive control, acid ascorbic was used at the same conditions [8].
Ferric reducing antioxidant power (FRAP)
Samples and ascorbic acid were used at different concentrations. 1mL of each sample was mixed with phosphate buffer (2.5mL, 0.2mol L-1, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5mL, 30mmol L-1). The mixture was incubated at 50 °C for 20min. A 2.5mL of TCA (0.6 mol L-1) was added to the mixture, which was then centrifuged for 10min at 3000g. The supernatant (2.5mL) was mixed with distilled water (2.5mL) and FeCl3 (0.5mL, 6 mmol L-1), and the absorbance was measured at 700nm [9].
DPPH- scavenging activity
A methanolic solution (50|iL) of each sample at different concentrations was added to 1.95mL of DPPH- (2,2-Diphenyl- 1-Picrylhydrazyl) solution (6x10-5M in methanol). The studied compounds were tested with methanol as control, BHA (Beta Hydroxy Acid), ascorbic acid and quercetin as antioxidant references. The absorbance at 515nm was determined after 30min. The absorbance (A) of the control and samples was measured, and the DPPH- scavenging activity (SA), in percentage, was determined as follow:
SA% = [(Acontrol-Asample)/Acontrol]x1001
IC50 (inhibition concentration of 50%) was obtained graphically from nonlinear regression analysis [10].
H2O2 scavenging activity
A solution of H2O2 (20mM) was prepared in phosphate buffered saline (PBS, 0.1M, pH 7.4). 1mL of sample or positive control (BHA and a-tocopherol) in methanol was added to 2mL of H2O2 solution in PBS. The absorbance was measured at 230nm, after 10min [11]. The percentage of H2O2 scavenged was calculated:
% of scavenged H2O2 = [(Acontrol-Asample)/Acontrol]x100
Hydroxyl scavenging assay
OH- radicals were generated from FeSO4 and H2O2, and detected by their ability to hydroxylate salicylate. The reaction mixture contained 1mL of FeSO4 (1.5mM), 0.7mL of H2O2 (6mM), 0.3mL of sodium salicylate (20mM) and different concentrations of organic extracts tested. After incubation for 1h at 37 °C, the absorbance was measured at 562nm. The percentage of scavenging effect was calculated as:
% of Scavenging = [1-(A1-A2)/xA0]x100
Where A0 was the absorbance of the control (without extract) and A1 was the absorbance in the presence of the extract and A2 was the absorbance without sodium salicylate. IC50 (inhibition concentration of 50%) was obtained graphically from nonlinear regression analysis [12].
β-Carotene bleaching method
A stock solution of β-carotene was prepared with 0.5mg of β-carotene in 1mL of chloroform, 25μL of linoleic acid and 200mg of Tween 40. The chloroform was evaporated and 100mL of oxygenated distilled water were added to the residue. 350μL of each sample were added to 2.5mL of the above mixture. The test tubes were incubated at 50 °C for 2h, together with two blanks, one contained BHA as a positive control and the other contained the same volume of methanol instead of the extracts. The absorbance was measured at 470nm. Antioxidant activities (inhibition percentages, I%) of the samples were calculated using the following equation:
I% = (A β-carotene after 2h assay/Ainitial β-carotene)x100
where A β-carotene after 2h assay is the absorbance of β-carotene after 2h assay remaining in the samples and A initial β-carotene is the absorbance of β-carotene at the beginning of the experiments. IC50 (inhibition concentration of 50%) was obtained graphically from nonlinear regression analysis [13]. All tests were carried out in triplicate and results were reported as means ±SD of triplicates.
Results and Discussion
Table 2: Yield of extraction, phenolic contents and antioxidant activity (DPPH- scavenging, OH- scavenging and p-carotene bleaching methods) of the different organic extracts of fruit part of Ammodaucus leucotrichus.
Methanol and ethanol extracts showed yields of the order of 10.02±0.49% and 9.92±0.28%, respectively (Table 2). An extraction yield of methanol extract (maceration) of Ammodaucus leucotrichus fruit part of about 20.4% was reported by Sifi et al. [14]. The contents of this plant on phenolics and flavonoids in their different organic extracts were important in polar organic extracts. The most important phenolic contents were observed in ethanol extract (160.61±6.28mg GAE/g). While the most important flavonoid contents were recorded in acetone extract (97.38±1.31mg CEQ/g). Chloroform extract contained the less important contents in those compounds (Table 2). Some species in Apiaceae family contain significant levels of phenolics and flavonoids. The study of Saeed et al. [15] on Torilis leptophylla methanolic extract reported high contents on those compounds (121.9±3.1mg GAE/g and 60.9±2.2mg RTE/g, respectively). Another study on three species of Apiaceae: Heracleum lasiopetalum Boiss, Kelussia odoratissima Mozaff and Echinophora platyloba DC, reported important levels of phenolics (from 74 to 120mg TAE/g), and flavonoids (from 7.63 to 14.52mg RE/g) [16].
The evaluation of antioxidant activity of the organic extracts was made by six conventional methods. The antioxidant activity of Apiaceae was investigated by several authors in the literature. Chandran et al. [17] investigated the antioxidant activity of aqueous and methanolic extracts of leaf part of Ardisia solanaceae Roxb. The methanolic extract of this plant revealed more antioxidant activity compared to the aqueous extract. Interesting antioxidant activity was reported by the authors of this publication [17]. Pirbalouti et al. [16] in their study on three species of Apiaceae family, endemic in Iran, reported an interesting antioxidant power of their methanol extracts tested by: the DPPH- test, reduction of iron and the ABTS- test.
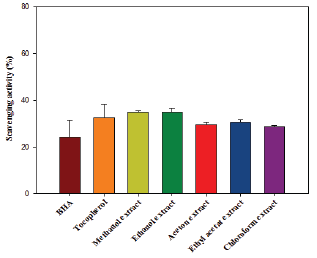
The antioxidant activity of organic extracts of the fruit part of Ammodaucus leucotrichus that we evaluated was moderate overall antioxidant tests used in our study (Figures 1-3). Extracts from polar solvents showed more antioxidant activity than those from non-polar one. In TAC test, we noticed that the methanol extract has shown the most important antioxidant activity compared to the other extracts organic, but lower than that of ascorbic acid. For DPPH- scavenging test, organic extracts: ethanol, methanol and acetone, had presented a moderate antioxidant activity with values of IC50 in the order of 29±1.85|tg/mL, 38.10±2.54|tg/mL and 41±0.56|tg/mL, respectively. Nevertheless, these extracts remain less active compared to the used positive controls. In H2O2 scavenging test, methanolic and ethanolic extracts presented an important activity superior to that of the positive controls used (BHA and tocopherol). The other extracts were also active. The chemistry behind this test is based on a transfer of electrons and proton between the antioxidant and hydrogen peroxide by reducing it in a water molecule. Measurement in the UV band makes this test very sensitive and estimate with a critical eye [18].
Figure 1: Total antioxidant activity of the different organic extracts of fruit part of Ammodaucus leucotrichus.
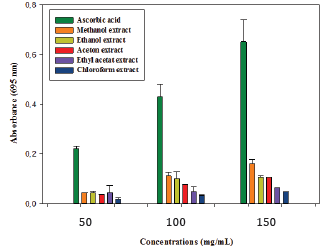
Figure 2: Reducing power activity (FRAP) of the different extract of fruit part of Ammodaucus leucotrichus.
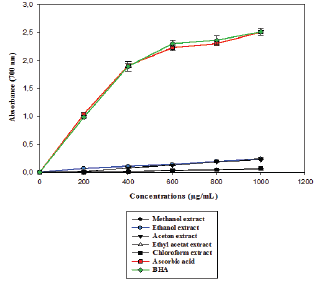
Figure 3: H2O2 scavenging activity of the different organic extracts of the fruit part of Ammodaucus leucotrichus.
Good antioxidant activity was recorded in OH- radicals scavenging test for methanolic extract, more interesting than the positive control (ascorbic acid). Assessment of the activity of an extract to scavenge the OH- radical is important, due to the high reactivity ofthis radical, which reacts with a wide range of molecules found in living cells (such as: sugars, lipids, proteins, nucleotides and so on). In the test of p-carotene bleaching, all organic extracts showed moderate activity and only the methanolic extract presented an IC50 (191.53±1.53|ig/mL) which is significantly lower than that of the BHA (4.26±0.98|ig/mL). In the FRAP test, all organic extracts showed very modest antioxidant activity.
Conclusion
Valorization of local flora is an important priority to find new interesting compounds that can be used in different fields. One of the endemic plants in Algeria, Ammodaucus leucotrichus was the subject of some scientific papers. In this study, we concluded that secondary metabolites from this plant exhibited a moderate antioxidant activity. These results help us to orientate our future studies in other directions of research.
Acknowledgment
The authors gratefully acknowledge Dr Hassani Fay^al (University of Tlemcen) for his help in the identification of the plant.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Financial Support
This work was supported by the Laboratory of Research in Natural Products, Faculty SNVSTU, University of Tlemcen, Algeria.
References
- Quezel P, Santa S (1963) New flora of Algeria and southern desert regions, CNRS, Paris, France.
- Benhouhou S (2005) A Guide to Medicinal plants in North Africa: database on Medicinal plants, IUCN center for Mediterranean cooperation, Malaga, Spain.
- Chehma A (2006) Catalog of spontaneous plants of the Algerian northern Sahara. Faculty of Sciences and Engineering Sciences, Protecting Ecosystems in Arid and Semi-Arid Zones Laboratory, University of Ouargla, Algeria.
- ElHaci IA, Bekhechi C, Bekkara FA, Mazari W, Gherib M, et al. (2014) Antimicrobial activity of Ammodaucus leucotrichus fruit oil from Algerian Sahara. Nat Prod Commun 9(5): 711-712.
- Hammiche V, Maiza K (2006) Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N'ajjer. J Ethnopharmacol 105(3): 358-367.
- Vermerris W, Nicholson R (2006) Isolation and identification of phenolic compounds, phenolic compound biochemistry. Springer, Dordrecht. pp.151-191.
- Ardestani A, Yazdanparast R (2007) Inhibitory effects of ethyl acetate extract of Teucrium polium on in vitro protein glycoxidation. Food Chem Toxicol 45(12): 2402-2411.
- Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2): 337-341.
- Yang J, Guo J, Yuan J (2008) In vitro antioxidant properties of rutin. Food Sci Technol 41: 1060-1066.
- Benhammou N, Bekkara AF, Kadifkova Panovska T (2009) Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplexhalimus. Compte rendu chimie 12(12): 1259-1266.
- Bhatia L, Bishonoi H, Chauhan P, Kinja K, Shailesh S (2011) In vitro comparative antioxidant activity of ethanolic extracts of Glycosmis pentaphylla and Bauhinia variegate. Recen Res Sci Technol 3(7): 1-3.
- Wang H, Gao XD, Zhou GC, Cai L, Yao WB (2008) In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias fruit. Food Chem 106(3): 888-895.
- Ebrahimabadi AH, Bidgoli DZ, Mazoochi A, Kashi FJ, Batooli H (2010) Essential oil composition, antioxidant and antimicrobial activity of the leaves and flowers of Chaerophyllum macropodum Boiss. Food Control 21(8): 1173-1178.
- Sifi I, Benaddou FZ, Yousfi M (2015) Antioxidant and antimicrobial activities of phenolic extracts of endemic plants Marrubium deserti and Ammodaucus leucotrichus from Algeria, World Academy of Science, Engineering and Technology International Journal of Biotechnology and Bioengineering, Algeria 9(1).
- Saeed N, Khan MR, Shabbir M (2012) Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complem Altern Med 12: 221-233.
- Pirbalouti AG, Setayesh M, Siahpoosh A, Mashayekhi H (2013) Antioxidant activity, total phenolic and flavonoids contents of three herbs used as condiments and additives in pickles products. Herba Polonica 59(3): 51-62.
- Chandran PR, Manju S, Vysakhi MV, Shaji PK, Achuthan Nair G (2013) In vitro antioxidant potential of methanolic and aqueous extracts of Ardisia solanacea Roxb. leaf J Pharm Res 6(5): 555-558.
- Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10(6): 1003-1008.
© 2018 Imad Abdelhamid El Haci, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






