- Submissions

Full Text
Archives of Blood Transfusion & Disorders
Prevalence of Hepatitis B Surface Antigen and Hepatitis B Core Antibody Among Prospective Blood Donors in Abuja, Nigeria
Ben Ikerionwu*
Nigeria
*Corresponding author: Ben Ikerionwu, Nigeria
Submission: January 08, 2018Published: November 27, 2018

ISSN 2578-0239Volume1 Issue4
Abstract
Background: Hepatitis B (HB) virus (HBV) remains a major risk factor for safe blood use. Compulsory pre-transfusion screening for HB core antibody (anti-HBc) to detect occult HBV infection (OHBVI) in HB surface antigen (HBsAg-)negative persons and prevent post-transfusion HBV infection in the target area is an unmet need. This study aimed to evaluate the presence of HBsAg and anti-HBc in prospective blood donors in Abuja, Nigeria and determine their association with age, gender, marital status (MS) and education.
Methods: We evaluated the presence of Total anti-HBc positivity and HBsAg-status among blood donors using the principle of enzyme-linked immunoassay (ELISA) method; and analysed their association with age, gender, MS and education using chi-squared (X2) test. The results were presented in simple tables and figures. A total of 300 individuals participated in the study. The p-value was set at P< 0.05 as significant level.
Result: Data for 300 participants were analysed with male: female dominant ratio of 24:1, recording prevalence of 7.7% (HBsAg+) and 17.7% (anti-HBc). Age group 25-34 years had the highest prevalence: 3.7% for HBsAg+ and 8.3% for anti-HBc; participants with formal education had higher prevalence for HBsAg+ (6.7%), anti-HBc (14.3%) than informal education (1.0% and 3.3%) respectively. Married participants recorded higher prevalence for HBsAg+(6.0%) and anti-HBc (14.0%) than unmarried participants (1.7% and 3.6%) respectively. Anti-HBc positive without HBsAg+ positivity was recorded among 10% of the participants. There was no significant association between prevalence of the markers and the demographic variables studied.
Conclusion: The high prevalence of anti-HBc (17.6%) among HBsAg+(7.7%) and (10%) among HBsAg-negative noticed in this study showed that the virus is actively replicating in chronic HBV carriers and persistently enhancing silent spread within the population in the target area.
Keywords: Prevalence; HBsAg; Anti-ssssHBC; Pre-transfusion screening; Abuja; Nigeria
Introduction
Blood transfusion service (BTS) is an integral and indispensable part of the healthcare system. It ensures safe, adequate and accessible blood supply when the need arises [1]. Blood borne pathogens including HBV may be transmitted through blood transfusion which can be potentially life threatening to those transfused. HBV infection constitutes a worldwide health threat with various levels of seriousness in different population. More than 350 million individuals have chronic, lifelong HBV infections and the World Health Organization (WHO) estimated that those who died globally in 2002 from acute and chronic liver disease due to HBV infection were more than 600,000 [2,3]. HBV is classified into eight genotypes: A-H [4] by phylogenetic analysis, using alignment of whole genome. Sub-genotypes with distinctive sequence characteristics and divergence in complete genome have been elucidated within the genotype A-C and F.
The difference in biological properties and heterogeneity in their global distribution may account for the differences in prevalence of HBV mutant in different geographic distribution [2,3]. However, protection against one subtype seems to confer immunity against the other, and no differences in clinical symptoms have been related to subtypes [5]. Nigeria is hyper-endemic for HBsAgpositivity; prevalence: (>8%) of the population [3,6,7]. A major route of spread is through blood. The infection can occur either as symptomatic disease or asymptomatic disease. Patients can clear the HBV and develop anti-HBs; but individuals who develop antibody to HBcAg are at risk of virus reactivation [7] showing persistence of virus, regardless of the time of infection or how high the titer of anti-HBs was [8] HBcAg is an HB viral protein which is an indicator of active viral reactivation and makes transmission easily possible. The core is considered “particulate,” not secreted, and does not circulate in the blood [9].
Reports have shown that ‘donors’ who are HBsAg-negative but positive for anti-HBc continue to replicate HBV even when such blood or human organs are transfused/given to recipients [10]; putting them at increased risk of continuous HBV infection. Therefore, knowing the significance of establishing HBV infection through serological testing and the clinical significance of anti-HBc in chronic-HBV infection is essential. There is limited data about the process of active and sustained HBV infectivity and infection in the target area; hence the need for complete routine screening of blood for HBV markers, including anti-HBc to aid identification of persons with high risk of transmission of the virus due to occult HBV infection (OHBVI) represented by the presence of HBV-DNA in serum and/or liver tissue in the absence of HBsAg positivity [11]; safe guide blood transfusion services and plan for management of patients. This study evaluated the prevalence of HBsAg and anti- HBc among blood donors in three healthcare centres in Abuja, Nigeria and their association with age, gender, marital status and education.
Materials and Methods
Study area
This was a prospective cross-sectional study; conducted in three hospitals in Abuja, the Capital City of Nigeria. The area has a population of about 2.153 million, a land mass of about 2,829 square miles (7,315 square yards) and lies on latitude 9.0667°N and longitude 7.4833°S [12]. It is bounded on the North, Southeast, South-west and West by states of Kaduna, Nasarawa, Kogi and Niger [13].
Sample size determination and sample selection
The sample size was determined using the formula:
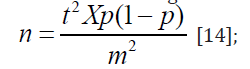
where n is the required sample size, t represented confidence level at 95% (standard value of 1.96), p represented 25% average prevalence of HBsAg+ in Nigeria from previous studies, m equaled 5% (standard value of 0.05) margin of error. With 4.16% (approximating to 12 donors) provided for dropouts or spilled samples, a total of 300 donor-samples was taken. Therefore, 300 blood samples were collected from prospective blood donors in three representative hospitals in the ratio of 169:78:103.
Inclusion/Exclusion Criteria
Included were prospective blood donors between the ages of 15-45 years of both sexes who were present at the selected hospitals at the time of study. They were asked to sign informed written consent. Excluded were blood donors below 15 years and above 45 years of both sexes and those who did not give their consent.
Data Collection Instrument-Pilot Testing
The questionnaire was first tested among 20 randomly selected health workers and hospital visitors to ascertain the validity of the data collection instrument. Prior to the collection of the blood samples, the piloted structured questionnaire was administered in order to obtain demographic information of the blood donors.
Blood Sample Collection
Blood samples were collected from prospective blood donors between June and September 2014. Two milliliter of blood sample was collected from each donor using standard venipuncture technique described by Ochei & Kolhatkar [15]. The blood samples collected were transferred into EDTA bottles and labeled accordingly for easy identification. The samples were transported to the serology laboratory at Fereprod Medical Centre, Abuja, for analysis.
Sample Preparation
The blood samples were centrifuged (Hackett Incorporation England, Model no:0029AB) at 1000000 revolution per minutes (rpm) for five minutes. The plasma from each sample was aspirated and transferred into a sterile sample bottle and stored at -20 °C prior to screening for further analysis [15].
Sample Screening and Interpretation
A commercially available ELISA kit (Advanced Quality Company China, Model; A1099. Batch B) designed for qualitative determination of HBsAg and Anti-HBc, was used to screen the blood samples. The assay was prepared according to the manufacture’s instruction in order to determine HBV positive samples. The one step test strip has a test region and a control region. Presence of a red coloured band in the test region indicates a positive result while the absence of a coloured band indicates a negative result. To ensure quality control, a coloured band will always appear at the control region while absence of this renders the result invalid.
Statistical Analysis
The data were tabulated and analysed using Statistical Package for Social Sciences (SPSS), version 20 software (SPSS Inc. Chicago, Illinois, USA). Categorical data were presented as numbers and percentages. Chi-squared (X2) test was used for associations between prevalence and categorical variables of age, gender, MS and education. The level of 0.05 (P< 0.05) was considered significant.
Result
Data for 300 participants were analysed with male, female dominant ratio of 24:1, recording prevalence of 7.7% (HBsAg+) and 17.7% (anti-HBc). The 12 females were HBsAg negative of which two were positive for anti-HBc (0.7%). Thirty (10%) participants tested positive for anti-HBc without testing positive for HBsAg. The descriptive prevalence distributions of HBsAg and Anti-HBc and demographic variable and their subsets; while the X2-squared statistical output of prevalence, P-value and correlation coefficient to ascertain the relationship between prevalence of HBV infection and demographic variables is shown in table 3. The distribution of blood donors according to age showed that the age group 25- 34 years had the highest prevalence of 3.7% for HBsAg and 8.3% for Anti-HBc followed by the age group 35-44 years which had a prevalence of 3.3% for HBsAg and 7.7% for Anti-HBc respectively.
The age group with the lowest positivity was observed among 15-24 years old, with a prevalence of 0.3% for HBsAg and Anti- HBc respectively. Unmarried blood donors recorded 1.7% for HBsAg and 3.6% for Anti-HBc, while 6.0% for HBsAg and 14.0% for anti-HBc were recorded among married blood donors. Blood donors without formal education had 1.0% for HBsAg and 3.3% for Anti-HBc, but blood donors with formal education recorded 6.7% prevalence for HBsAg and 14.3% for Anti-HBc. The mono-positivity and co-positivity pattern of HBsAg and anti-HBc markers showed that 30 (10%) participants were positive for anti-HBc without positivity for HBsAg. Despite the differences in prevalence, chisquared statistical analysis revealed no significant relationship between prevalence of HBV infection and the studied demographic variables of the blood donors.
Discussion
When infection of HBV occurs, there follows the development of host immunity, the nature determines the course and type of disease and its consequences; some will develop persistent HBsAg+ while others will clear the virus and become HBsAg-negative [16]. However, studies have shown that some HBsAg-negative individuals with anti-HBc or circulating HBV-DNA or both could remain infectious [17,18] and continue to spread the infection through HBsAg-negative-anti-HBc positive donors [19]. Anti-HBc is the first antibody produced after HBV infection and the only detectable marker in the window period. The isolation of anti-HBc in serum in the absence of HBsAg may be due to resolved HBV infection which HBsAb becomes undetectable. The prevalence of HBV infection in this study is 7.7% and 17.7% for HBsAg and anti-HBc respectively, aligning with the placement of Nigeria as hyper-endemic country for CHB disease [20]. Studies conducted in different countries have shown in-patients undergoing haemodialysis and blood donotion to have prevalence of 11.5%, 16.6%, 17%, 42%, 48% and 49.1% for anti-HBc [21-30].
Mono-positivity and co-positivity pattern of HBsAg and anti- HBc observed in this study revealed that positivity to anti-HBc alone were observed in 10% of the blood donors. There could be false positive among anti-HBc-positives in HBsAg-negative individuals which can occur in low risk of past HBV infection or distantly resolved HBV infection in an area of endemicity such as the target area. However, using polymerase chain reaction (PCR) method to detect HBV-DNA in serum could help to resolve this doubt. Also, when protein mutation occurs, it may be undetectable during the window period or chronic infection in the absence of HBsAg if poor sensitive diagnostic assay was used [31]. Donors who were HBsAg-negative but have occult HB infection detectable only by anti-HBc are potential sources of HBV infection [32]. In a study in Sao Paulo about post-immunization screening concluded that testing for isolated hepatitis B core antibody was significant in the screening of blood donors Almeida [33]. In line with this study, results of studies of organ transplantation have documented reactivation of HBV in HBsAg-negative-anti-HBc-positive patients [8,33,34]. Therefore, it is suggested that this category of individuals should be regarded as being at risk of reactivation of HBV who could benefit from counseling. The no-correlation (P>0.05) observed in this study between prevalence and demographic variables (age, gender, marital status and education) was similar to the findings of Japhet and Adekeye [35,30]. In summary, following the methods currently employed in the target area for pre-transfusion blood screening, this study has been able to evaluate the prevalence of anti-HBc+ along with HBsAg-status among blood donors and noted the necessity for both to be used as discriminatory markers for the risk of post-transfusion transmission of HBV.
Limitations and Strengths of the Study
The protocol for this study did not include counseling of donors who tested positive for anti-HBc and are at risk of transmitting HBV to their contacts. Also, the study was not designed to include determination of the phase of infection or other sources of contracting the HBV to impart treatment and prevention. Being a cross-sectional study, participants who were anti-HBc were not followed up to check those who may have developed liver complications. Longitudinal follow-up study is needed to examine the clinical implication of being anti-HBc positive. The screening method (ELISA) used may not detect HBsAg-positive individuals during the Window period. Nevertheless, the high sample size employed and the provision made for dropouts and blood sample spills were reasonable enough to give strength to the outcome of this study.
Conclusion
Positive HBsAg individuals are at increased risk of chronic hepatitis B and liver diseases: cirrhosis, hepatocellular carcinoma (HCC) including death. In the target area, the presence of HBsAg is the major diagnostic screening employed to determine HBV infection among prospective blood donors; anti-HBc is not used as a marker to determine previous exposure to HBV. This study reveals that HBV continues to be a major health burden even after adopting HBsAg as a marker for the screening of blood donors and notes that HBc Antigen (HBcAg) plays a role in the persistent propagation of the virus. We therefore suggest the mandatory inclusion of anti-HBc marker for the screening of blood donors to rule out the risk of post-transfusion HBV infectivity even in the presence of HBsAg-negativity. Awareness should be enhanced by public and private healthcare stakeholders about the danger posed by poorly screened blood.
Ethical Clearance
Ethical approval was obtained from the Federal Capital Territory (FCT) Health Research Ethics Committee, Abuja.
Informed Consent
Participation was voluntary and informed written consent was obtained from participants before the study.
References
- Islam MB (2009) Blood transfusion services in Bangladesh. Asian J Trans Sci 3(2): 108-110.
- Norder H, Hammas B, Lofdahl S, Courouce AM, Magnius LO (1992) Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J Gen Virol 73(pt 5): 1201-1208.
- Mulders MN, Oyedele OI, Ola SO, Odaibo GN, Olaleye DO, et al. (2001) Phylogenetic analysis of new hepatitis B virus isolates from Nigeria supports endemicity of genotype E in West Africa. J Med Virol 65(3): 463-469.
- Nordor H, Courouce AM, Coursaget P, Echevarria JM, Lee SD, et al. (2004) Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology 47(6): 289-309.
- Elbedewy TA, Elshweikh SA, Bajomy N (2016) Prevalence and significance of hepatitis-B core antibodies among hepatitis B surface antigen-negative Egyptian patients on hemodialysis in Al-Gharbia governorate. Tanta Med J 44(2): 33-38.
- Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, et al. (2009) Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 9(2): 98-108.
- World Health Organization Hepatitis B vaccine (2004) Weekly Epidemiological Record 79(28): 253-64.
- Blanpain C, Knoop C, Delforge MI, Antoine M, Peny MO, et al. (1998) Reactivation of hepatitis B after transplantation in patients with preexisting anti-hepatitis B surface antigen antibodies: report on three cases and review of the literature. Transplantation 66(7): 883-886.
- Yuan Q, Song LW, Cavallone D, Moriconi F, Cherubin B, et al. (2005) Total hepatitis B core antigen antibody, a quantitative non-invasive marker of hepatitis B virus Induced liver disease. PLoS ONE 10(6): e0130209.
- Behzad BA, Mafi NA, Tabei SZ, Bagheri LK, Rashidi M, et al. (2005) Indication of anti-HBc screening. J Med Sci 30(1): 28-33.
- Allain JP (2005) Occult hepatitis B virus infection. Gastroenterology 2(1): 14-30.
- (2006) Federal Capital Territory, Nigerian Census.
- Abuja Maps (2017) Google map data.
- Bruce N, Pope D, Stanistreet D (2008) Quantitative methods for health research: A practical interactive guide to epidemiology and statistics. England, UK.
- Ochei J, Kolhatkar A (2000) Medical laboratory science: Theory and practice. McGraw-Hill, USA.
- Fabrizi F, Martin P, Ponticelli C (2002) Hepatitis B virus and renal transportation. Transplant Rev (Orlando) 25(3): 102-109.
- Rehermann B, Ferrari C, Pasquinelli C, Chisari FV (1996) The hepatitis B virus persists for decades after patient’s recovery from acute hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 2(10): 1104-1108.
- James L, Bailey, Balter P, Berns J, Butera E, et al. (2001) Recommendations for preventing transmission of infections among chronic hemodialysis patients. Mobidity and Mortality Weekly Report. Centers for Disease Control and Prevention 50(5): 1-43.
- Wachs ME, Amend WJ, Ascher NI, Bretan PN, Emond J, et al. (1995) The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation 59(4): 230-234.
- Forbi JC, Vaughan G, Purdy MA, Campo DS, Xia GI, et al. (2010) Epidemic history and evolutionary dynamics of hepatitis B virus infection in two remote communities in rural Nigeria. PLoS One 5(7): 5e11615.
- Ismail H, Soliman M, Ismail N (2010) Occult hepatitis B virus infection in Egyptian hemodialysis patients with or without hepatitis C virus infection. Pathology and Laboratory Medicine International Volume 2: 113-120.
- Helaly GF, El Ghazzawi EF, Shawky SM, Farag FM (2015) Occult hepatitis B virus infection among chronic hemodialysis patients in Alexandria, Egypt. J Infect Public Health 8(6): 562-569.
- Mandour M, Nemr N, Shehata A, Kishk R, Badran D (2015) Occult HBV infection status among chronic hepatitis C and hemodialysis patients in Northeastern Egypt: regional and national overview. Rev Soc Bras Med Trop 48(3): 258-264.
- Said ZN, Sayed MH, Salama II, Aboel Magd EK, Mahmoud MH, et al. (2013) Occult hepatitis B virus infection among Egyptian blood donors. World J Hepatol 5(2): 64-73.
- Oluyinka OO, Tong HV, Bui Tien S, Fagbami AH, Adekanle O, et al. (2015) Occult hepatitis B virus infection in Nigerian Blood donors and hepatitis B virus transmission risks. PLoS One 10(7): e0131912.
- Abd El Kader Mahmoud O, Abd El Rahim Ghazal A, El Sayed Metwally D, Elnour AM, Yousif GE (2013) Detection of occult hepatitis B virus infection among blood donors in Sudan. J Egyptian Pub H Association 88(1): 14-18.
- Uneke CJ, Ogbu O, Inyamu PU, Anyanwu GI, Njoku MO, et al. (2005) Prevalence of hepatitis B surface antigen among blood donors and human immunodeficiency virus-infected patients in Jos, Nigeria. Mem Inst Oswaldo Cruz 100(1): 13-16.
- Alao O, Okwori E, Egwu C, Audu F (2009) Seroprevalence of hepatitis B surface antigen among prospective blood donors In an urban area of Benue State. The Internet J Hematol 5(2).
- Olokoba AB, Salawu FK, Danburam A, Desalu OO, Olokoba LB, et al. (2009) Viral hepatitis in voluntary blood donors in Yola, Nigeria. European J Sci Res 31(3): 329-334.
- Adekeye AM, Chukwuedo AA, Zhakom PN, Yakubu RS (2013) Prevalence of hepatitis B and C among blood donors in Jos South LGA, Plateau State, Nigeria. Asian Journal of Medical Sciences 5(5): 101-104.
- Almeida NC, Strauss E, Sabino EC, Sucupira MC, Chamone DA (2001) Significance of isolated hepatitis B core antibody in blood donors from São Paulo. Rev Inst Med Trop Sao Paulo 43(4): 203-208.
- Kleinman SH, Kuhns MC, Todd DS, Glynn SA, McNamara A, et al. (2003) Retrovirus epidemiology donor study presence of anti-HBc: Implications for transfusion transmission and donor screening. Transfusion 43(6): 696-704.
- Marcellin P, Giostra E, Martinol PM, Loriot MA, Degos F, et al. (1991) Redevelopment of hepatitis B surface antigen after renal transplantation. Gastroenterology 100(5 Pt 1): 1432-1434.
- Minuk GY, Sun DF, Greenberg R, Zhang M, Hawkins K, et al. (2004) Occult hepatitis B virus infection in a North American adult hemodialysis patient population. Hepatology 40(5): 1072-1077.
- Japhet OM, Adesina OA, Donbraye E, Adewumi MO (2011) Hepatitis B core IgM antibody (anti-Hbc IgM) among Hepatitis B Surface Antigen (HBsAg) negative blood donors in Nigeria. Virology J 8: 513-514.
© 2018 Ben Ikerionwu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






