- Submissions

Full Text
Archives of Blood Transfusion & Disorders
Feature of Older Patients with Sickle Cell Disease in the Congo
Ngolet LO*, Malanda F, Ockouango Guelongo Ova JD and Elira Dokekias A
Clinical Hematology Unit, Marien Ngouabi University, Congo
*Corresponding author: Ngolet LO, Clinical Hematology Unit, Marien Ngouabi University, Brazzaville, Republic of Congo. BP: 32, Republic of Congo, Congo
Submission: September 18, 2017;Published: February 26, 2018
ISSN: 2578-0239Volume1 Issue2
Abstract
Background: Despite the high mortality rate of patients with sickle cell disease during the childhood in Africa, their life expectancy is increasing. Consequently, the number of adults is growing. Hence, health providers need to be aware about their clinical and biological pattern.
Patients and Methods: We studied clinical and biological aspects of 47 patients with sickle cell disease and phenotype SS aged 40 years and over from 2011 to 2016.
Results: The mean age was 45 (range: 40-59) years. The female population was older with a mean age of 47 (p=0.05). 23 over 47 (48.9%) had a low monthly income. Older adults presented sever sickle cell related acute morbidity, with a number of 1.83 vasoocclusive crisis, 2 anemic crisis/ blood transfusions and 1.59 admission episodes per year blood transfusion procedure episodes. In our group 57.44% developed a chronic complication. The most frequent complications of older patients with sickle cell disease were elevated tricuspid regurgitant jet velocity on echocardiogram performed on 12/47 patients, avascular necrosis of the femoral head (19.14%) and persistent leg ulcer (8.51%). The mean hemoglobin rate was higher than the rest of the sickle cell disease population.
Conclusion: Sickle cell related acute morbidity is high in older patients. Chronic complications are more frequent in this group and elevated regurgitant jet velocity more common finding.
Keywords: Sickle cell; Congo; Older adults; Morbidity
Introduction
Sickle cell disease is the most common inherited blood disorder in the Sub-Saharan where 75% of the 300,000 births worldwide live [1]. Few aspects of the sickle cell in the adulthood are known in the region because the mortality in the childhood is high. However despite our low resources, a slight decline in the mortality rate of SCD patients in Africa has been noticed due to improved supportive care such as malaria prophylaxis, SCD awareness, earlier diagnosis and management care [2]. Consequently, childhood survival has improved and adult population with sickle cell disease is growing with the spectrum of clinical aspects that are for some unknown [3]. Reports of the adults’ natural history are spares and clinical aspects are unknown in the continent [4,5]. One study of homozygous HbSS SCD patients over 30 years of age was performed In the Congo. Thus we undertook to study SCD patients over 40 years of age.
Patients and Methods
Data collection and assessment procedure
We recruited through outpatient clinic in the hematology department HbSS SCD adult patients 40 years over of age .This study was carried from 2011 to 2016. Data were obtained from adult individuals who were receiving their care from the hematology department or new patients that consulted for sickness. Patients with sickle cell β0 and SC genotypes were excluded in the study.
All patients provided informed consent in accordance with the Declaration of Helsinski. Research protocol was approved by the local institution ethic board (CERSSA). Each patient was interviewed and data were collected in the SCD medical history form. We obtained the following data:
A. Sociodemographic:
- Age
- Gender
- Employment status
- Income. The income of the family was interpreted based on the official lowest salary fixed by the Congolese government (XAF 90,000/ USD 153.53). The income was low when it was lower than XAF 90,000/ USD 153,53, middle when it was between than XAF 90,000/ USD 153,53 and XAF 500,000/ USD 852.96 and high when it was above XAF 500,000/ USD 852.96.
- Number of children per family
- Number of miscarriage SCD related for female patients
B. Morbidity
i. The number of crisis, hospitalization admissions and blood transfusion procedures that occurred during the study period.
C. We also recorded chronic complications SCD related noticed at the time of the inclusion in the study as leg ulcer, avascular femoral necrosis and Gall Bladder. Chronic complications were either obtained from patients’ medical records or diagnosed at the time of the visit in the hematology department for symptomatic patients.
D. Heart ultrasound was performed on symptomatic patients that were presenting a congestive heart failure or dyspnea. Tricuspid regurgitant jet velocity was considered elevated (ETRJV) when the velocity was above 2.5m/sec as per cu
5 milliliters of blood for complete blood count and 5 milliliters for analysis of LDH were sampled for each patient. Samples were obtained on patients in stationary period (no painful or anemic crisis) for at least 3 months. Painful crisis was defined as an unpredictable intense pain that generally lasts hours to a few days.
We defined anemic crisis was defined by a decrease of at least 2g/dL from the steady state hemoglobin hemoglobin The most common process leading to this complication in adult patients is the Hyperhemolysis crisis. Transient red cell aplasia and acute splenic sequestration are other causes of anemic crisis and are more frequent in children.
Blood transfusion was indicated when the hemoglobin rate was below 6g/dL of the patient or if there is sufficient physiological derangement such as: heart failure, dyspnea, hypotension or marked fatique related to hemoglobin concentration fall. A total of sixty two patients over 40 years of age consulted during the study period. Among them 47 (75.81%) agreed to participate to the study.
Statistical Analysis
For the description of each quantitative variable, the mean, range, frequencies and percentages were calculated. Student’s test was used for the comparison of variable. A significance level of p≤0.05 was considered.
Results
Sociodemographic
47 patients over 40 years of age and were identified, of which 17 (36.2%) were males while 30 (63.8%) were females. The median age of the population was 45 years (extremes: 40 and 59 years, X2, p=0.04). Female patients had higher median age 47 years (extreme: 40 and 59 years, X2=17.05, p=0.05) compared to male ones: 44 years (extreme 40 and 57 years, X2 =14.65, p=0.10). Twenty five were unemployed (53.2%) in which 19 females (76%) and 6 males (24%). The total monthly income was low for 23 patients (48.9%), middle for 15 (31.9%) and high for 9 participants (19.1%), p=0.04. The low income was more frequent in the female population (58.3%) than the male one (p= 0.02). The average number of children per family was 0.5 for SCD women versus 1.6 for < male (p=0.01). Sixteen women over 30 (53.3%) reported one episode of miscarriage related to sickle cell.
Clinical history
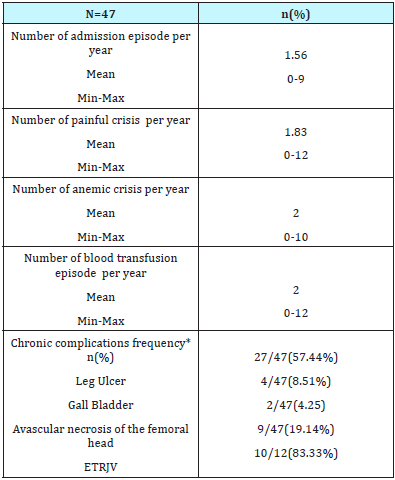
The clinical characteristics of SCD patients over age 40 are described in the Table 1.
Table 1: Clinical criteria
Biological characteristics
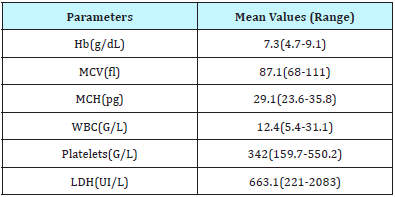
Laboratory determinants of older adult patients with SCD are reported in the Table 2.
Table 2: Biological criteria
Discussion
The present paper describes the clinical and biological features of SCD patients over 40 years of age. We acknowledge on a series of limitation in our study. First, SCD renal, eye abnormalities, brain imaging, pulmonary evaluation and infectious complications have not been documented due to lake of diagnosis facilities and lake of funding. Second, we did not study the co-morbidities. They may in this range of age, additionally to SCD, influence the length of life expectancy. Third, the cross sectional did not give us tools to elucidate why this population survives longer.
The median age of the population studied was 45 years. The female population was older with a mean age of 47 years. This epidemiological distribution is a picture of the demographics in the Congo and is not relative to any inherent biological characteristics. Manpreed et al. [3] in a similar study in the USA found, an older population with a median age of 49 years. The longer life expectancy, in Western countries, is partially the result of a systematic universal newborn screening (NBS), pneumococcal infection prevention, early detection of complications as stroke and more recently, use of Hydroxyurea in adult [7-9]. Despite the lake of such interventions in Africa, a slight decrease of the childhood mortality has been noticed due to a better management of the disease, annual SCD awareness and malaria prophylaxis. Consequently, life expectancy of patients with SCD is increasing. 19.1 and 31.9%, have respectively high and middle income which is higher that the demographic distribution of the population in the Congo [10]. Tolo-Diebkile in Ivory Coast noted the same distribution [5]. In low resources countries where there is not any medical coverage system, the level of income is proportionally linked to quality and accessibility of care, thus outcome and length or survival [4,5]. The longer survival is also determined by the Hb rate level that influences the severity of the disease [11- 13]. Low hemoglobin rate is associated with high morbidity and mortality of SCD [11-13]. In our series, the mean of hemoglobin (7.3g/dl) was higher than the average of the younger Congolese SCD population [4,5,14].
It seems that our patients are more symptomatic compared to other studies. Losada et al in Trinidad reported that 72% of their cohort did not develop any acute crisis in the past two years while in our series, 75% had developed one [15]. Surprisingly, Manpreet et al. [3] in the US, documented higher frequency of vaso occlusive crisis and hospitalization admission episodes. The diversity of the genotypes included in the studies does not allow us to make consistent comparison.
Chronic complications are reported to be more common in older adults and ETRJV seems to constitute the most frequent chronic complication [3,16]. Because the Heart ultrasound was underwent only on 12 patients over 47, we are unable to give an accurate estimation of the vasculopathie. However, the morbidity was found on 10/12 (83.33%) which may presume a high incidence of the ETRV in our cohort.
Conclusion
SCD survivor rate in Africa is growing up and clinical and biological characteristics need to be known and better understand. Genetics characteristics and other factors may explain longer survival that request further studies.
References
- World Health Organization (2006) Management of birth defects and haemoglobin disorders: report of a joint WHO-March of Dimes Meeting. Geneva: World Health Organization.
- Grosse SD, Obame I, Atrash H, Amendah DD, Piel FB, et al. (2011) Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med 41(6 Suppl 4): S398-S405.
- Manpreet KS, Cohen A (2015) Aging in sickle cell disease: co-morbidities and new issues in management. Hemoglobin 39(4) : 221-224.
- Elira Dokekias A, Nzingoula S (2001) Profil du drépanocytaire homozygote après l’âge de 30 ans. Med d’Af Noire 48(10): 411-418.
- Tolo Diebkilé A, Koffi Kouassy G, Sawadogo D, Kouakou B, Siransy Bogui L, et al. (2010) Drepanocytose homozygote chez l’adulte ivoirien de plus de 21 ans. Cahiers Santé 20(2): 63-67.
- Reeves ST, Glas KE, Eltzchig H, Pathew JP, Rubenson DS, et al. (2007) Guidelines for performing a comprehensive epicardial echocardiography examination: recommendations of the American Society of Echocardiography and the society of Cardiovascular Anesthesiologists. Anest Analg 105(1): 22-28.
- Morris J, Dunn D, Beckford M, Grandison Y, Mason K, et al. (1991) The hematology of homozygous SCD after the age of 40 years. Br J Haematol 77(3): 382-385.
- Telfer P, Coen P, Chakravorty S, Wilkey O, Evans J, et al. (2007) Clinical outcomes in children with sickle cell disease in living in England: a neonatal cohort in East London. Haematologica 92(7): 905-912.
- Steinberg MH, Barton F, Castro O, Pegelow CH, Dallas SK, et al. (2003) Effect of hydroyurea on mortality and morbidity in adult sickle cell anemia. J Am Med Assoc 289(13): 1645-1651.
- A propos du PNUD en Republique du Congo.
- Redelsperger MM, Bardakdjlan Michau J, Neonato MG, Girot R (2003) Diagnostic biologique des syndromes drépanocytaires. In: Girot R, Begué P, Galacteros F (Eds.), La drépanocytose. John Libbey eutrotext, France, pp. 13-29.
- Nagel RL, Rao SK, Dundq Belkhodja Q, Connolly MM, Fabry ME, et al. (1987) The hematologic characteristics of sickle cell anemia bearing the Bantu haplotypes: the relationship between (G) γ and HbF level. Blood 69(4): 1026-1030.
- Tshilolo L, Summa V, Gregory C, Kinsiama C, Bazeboso JA, et, al. (2012) Fotal haemoglobin, erythrocytes containing foetal haemoglobin, and hematological features in Congolese patients with sickle cell anaemia. Anemia 6(2): 1-6.
- Kocko I, Ngolet LO, Galiba Atipo FO, Okco LT, Malanda F, et al. (2016) Valeurs de l’hémogramme du drépanocytaire adulte Congolais en période intercritique. HSD 17(4): 63-66.
- LosadaR, Bravo I, Capildeo K, Charles K (2006) Is the group of older sickle cell patients from Trinidad and Tobago different? Am J Hematol 81(3): 219-220.
- Mc Kerrel TDH, Cohen HW, Billet HH (2004) The older sickle cell patient. Am J Hematol 76(2): 101-106.
© 2017 Ngolet LO, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






