- Submissions

Full Text
Archives of Blood Transfusion & Disorders
Evaluation of Liver Iron Concentrations in Children with Beta Thalassemia Infected with Hepatitis C Virus Before and After Spirulina Therapy by Magnetic Resonance Imaging
El Shanshory MR1*, Awad MA1, El Shafey RA2, Soliman HH3 and Alsharkawy AA4
1Department of Pediatrics, Tanta University, Egypt
2Department of Radiology, Tanta University, Egypt
3Department of Tropical Medicine, Tanta University, Egypt
4Department of Pediatrics, Ministry of Health, Tanta University, Egypt
*Corresponding author: Mohamed Ramadan El Shanshory, Professor of Pediatrics, Chairman of Hematology & Oncology Unit, Pediatric Department, Faculty of Medicine, Tanta University, Egypt
Submission: September 13, 2017 Published: October 24, 2017
ISSN: 2578-0239Volume1 Issue1
Abstract
Background: Magnetic resonance imaging (MRI) assessment of liver iron concentration (LIC) is necessary for the quantitative staging of iron overload in children with β-Thalassemia. There is no enough evidence about the effect of spirulina therapy on LIC.
Aim of the work: To assess LIC by MRI in multitransfused β-Thalassemic children infected with HCV before and after Spirulina Therapy
Patients and methods: Thirty multi-transfused β-thalassemic children infected with HCV, were subjected to clinical evaluation, appropriate laboratory investigations and assessment of LIC by MRI. They were classified according to LIC into mild (group 1) and moderate to severe group (group 2). In addition to standard packed red cell transfusion, Spirulina therapy was given orally for 3 months, after which re-evaluation of these children was performed by repeating the same investigations.
Results: There were significant increases in LIC associated with significant changes in other MRI parameters (significant decrease in T2* and significant increase in R2*) in patients with β-Thalassemia of moderate to severe group as compared to those of the mild group before treatment. The mean values of serum ferritin (SF) were insignificantly higher among patients of mild group. There was no correlation between different MRI parameters and SF level. There were negative correlations between LIC and T2* and positive correlation between LIC and R2*. There was a significant decrease in values of LIC accompanied with significant improvements in SF after spirulina therapy as compared to their pretreatment values in patients of the moderate to severe group.
Conclusion: Spirulina therapy may have favorable effects on lowering the values of LIC in children with β-Thalassemia infected with HCV.
Keywords: Childhood; Iron overload; MRI; Thalassemia
Introduction
The thalassemias are a heterogeneous group of genetic heritable disorders of hemoglobin (Hb) synthesis, considered as the most common monogenic disorder in the world, affecting both sexes equally and poses a severe health and economic burden to patients and families at risk [1]. Thalassemia is a major health problem in Egypt since it is estimated out of 1.5million live births, 1000 children with thalassemia are born annually [2].
Multicenter cross-sectional studies have reported that the development and the severity of liver fibrosis are strongly related to the extent of the liver iron overload and to the presence of chronic HCV infection [3]. Hepatitis virus C infection is the main risk factor for liver fibrosis in transfusion-dependent thalassemia. Excess liver iron is now clearly recognized as a cofactor for the development of advanced fibrosis in patients with HCV infection [4]. Despite its clinical relevance, thalassemia-associated liver damage has been insufficiently characterized [5].
The current gold standard to quantify iron overload is nontargeted percutaneous liver biopsy and subsequent measurement of liver iron concentration (LIC) in biopsy specimens using atomic absorption spectrophotometry [6], which may not be representative of the entire liver, particularly in cases of heterogeneous iron deposition [7]. The invasiveness of biopsy is a limitation for all patients due to discomfort, anxiety, and rare complications including death. Due to these limitations of liver biopsy, there is a clinical need for noninvasive iron quantification techniques [6].
Serum markers such as serum iron, transferrin, and ferritin can be assessed through venipuncture, and provide the simplest and least expensive method to assess body iron stores. Nonetheless, serum iron markers are often inaccurate and, because they are acute phase reactants, can be confounded by systemic conditions such as malignancy or inflammatory states [6]. In contrast, Magnetic Resonance Imaging (MRI) represents the most available noninvasive technique to assess the hepatic iron content and shows a good correlation with biopsy results [8,9]. It is the best noninvasive method for measuring the level of iron in the liver for confirming the diagnosis, determining the severity and monitoring therapy with high sensitivity, specificity, and positive and negative predictive values [10].
Spirulina (blue-green algae), a filamentous cyanobacterium, possesses diverse nutritional and health benefits due to high concentrations of nutrients. It is rich in protein, vitamins, minerals and antioxidants, and is often considered to be one of the most nutritious foods available including protection of the liver [11,12]. The Spirulina treatment resulted in no side effects among the patients [13,14] which promotes investigators to conduct clinical trials to test the safety and efficacy of this supplement in patients with chronic hepatitis C [15]. The hepatoprotective effect of Spirulina is attributed to its anti-inflammatory and antioxidant effects that can produce up regulation of antioxidant enzymes, provocation of a free radical scavenging enzyme system and excretion of iron from the body by effective chelation [13,16,17]. The antioxidant effect of Spirulina is attributed to some active constituents that include: β-carotene, vitamins C and E, enzyme superoxide dismutase (SOD), selenium, metallothioneins and brilliant-blue polypeptide pigment phycocyanin [18]. The high Spirulina content of the enzyme SOD plays an important role in the elimination of reactive oxygen species derived from the peroxidative process in liver tissues. Moreover, SOD removes superoxide by converting it to H2O2, which can be rapidly converted to water by Catalase [19,20].
Furthermore, it was reported that the iron ions decreased and the lipid peroxidation process was inhibited by phycocyanin isolated from this microalga [16]. The aim of this study is to assess liver iron concentration by magnetic resonance imaging for multitransfused β-Thalassemic children infected with HCV before and after Spirulina Therapy.
Patients and Methods
This study was carried out upon 30 randomized selected multi-transfused β-thalassaemic children from 125 infected with hepatitis C virus (HCV) that were diagnosed by serological detection of HCV-Ab, and detection of serum HCV RNA by polymerase chain reaction (PCR). These children were selected from those attending outpatient clinics at the Hematology Unit, Pediatric Department, Tanta University Hospital during the period from December 2014 to December 2015.
This is a randomized clinical trial was approved by the Ethical Committee of the Faculty of Medicine, Tanta University. The patients were enrolled after obtaining an informed consent from their guardians. The study was registered in a Clinical Trial. Gov under ID: NCT02744560. Children with β-thalassemia less than 3 years old, with liver decompensation, with viral hepatitis B, and in whom MRI was contraindicated to perform as patients with intraocular metallic foreign body, with cardiac pacemakers, and who refused MRI examination were excluded from the study.
All patients with thalassemia undergo: Complete history taking with special emphasis on: Age, sex, order of birth, consanguineous marriage, disease duration, family history of chronic hemolytic anemia and transfusion history (age of first blood transfusion, frequency and dose of transfusion per month), chelation history (age at start of chelation therapy, type and dose of chelating agents, compliance of patient to the treatment, complications) and history of splenectomy (its indication, age at the time of operation). And full examination: Anthropometric measures (weight, height & head circumference), chest, heart, abdominal (liver, spleen and ascites) and neurological assessment were performed. The following laboratory investigations were done: Complete blood count (CBC), serum ferritin level (SF),Liver function tests including: Total and direct serum bilirubin, liver enzymes (ALT, AST), total serum proteins, serum albumin, as well as prothrombin time (PT) and activity.
Radiological evaluation of liver iron concentration measurement by MRI for the assessment of liver iron content using T2 gradient echo pulse sequence in the axial plane. Examination was done for all patients in MR unit, Radiology and Imaging Department, Tanta university Hospital, using 1.5 tesla system (Toshiba, GE medical system).
Preparation
Before entering the examination room, the patients and their parents were instructed to remove all ferromagnetic objects like metallic objects. Patients were instructed about the importance of being calm with no motion throughout the time of examination.
Technique
Multiple axial gradient images with the following Parameters: TE (1.7/2.2/ 3/3.5/4/5/5.5/8/9/10/12/15) milliseconds, TR =20msec/echo time, Flip angle =20, Field of view (FOV) =40x38mm, Matrix (frequency x phase) =128 x 192 pixels, Slice thickness =10mm with no gap interval.
Items needed for analysis of MRI images were:
A. Spread sheet program that supports an MS-ECel file.
B. Software to draw regions of interest (ROIs) in the original MRI images and this is a standard in any MRI scanner.
C. Images were acquired in the scanner and imported into software for ROI drawing.
i. Major vessels of liver were avoided and no need to cover the whole liver but it was sufficient to cover a large part of the right lobe.
ii. Each image generated a pair of numbers: the TE (echo time) and the mean signal intensity (SI) of the drawn ROI.
D. The TEs and ROI values were inserted in the two columns of the spread sheet.
E. As the examiner recorded the numbers, the graph and some values changed automatically.
The points were updated and the fitting line drawn automatically T2*, R2* and LIC were calculated automatically [21].
In addition to classic transfusion, Spirulina (GNC Natural Brand Spirulina), 500mg, Capsules, by General Nutrition Corporation, USA) in a dose of 250mg/Kg/day by the oral route was given to thirty patients with thalassemia infected with HCV.
Follow up of these patients was performed for 3 months. Reevaluation of these children after 3 months of Spirulina treatment was performed by repeating the previous clinical assessment and laboratory investigations.
Statistical Analysis
Quantitative data were presented as mean±standard deviation. The independent t-test was used to compare the means of the two groups. Qualitative data were presented as count and appropriate proportion. The chi-square test was used to compare the two independent proportions. Pearson’s correlation coefficient was used to test the association between two variables. Significant results were considered with P≤0.05. SPSS (Statistical Package for Social Sciences) version 13 was used in data entry and analysis (IBM Corp, Armonk, NY, USA).
Results
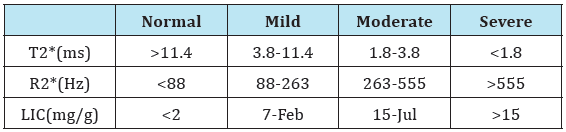
HCV-infected patients with thalassemia enrolled in this study were classified before Spirulina therapy, according to the estimation of LIC by MRI into mild group (n=18, 60%) and moderate to severe group (n=12, 40%) according to (Table 1) [22]. There were no significant differences between patients’ groups as regard to age, sex, and blood transfusion. There were no significant differences between patients’ groups as regard all of anthropometric measures and clinical data.
Table 1: Ranges of different MRI parameters for measurement of LIC as reference [22].
There was significant elevations of ALT and AST levels in moderate to severe group compared to the mild group with no significant differences between both groups as regard other laboratory data. It is to be noted that, no significant changes in the mean values of serum ferritin levels between patients’ groups, but the mean values of serum ferritin were insignificantly higher among patients of mild group (Table 2).
Table 2: Laboratory data in patients’ groups before treatment.
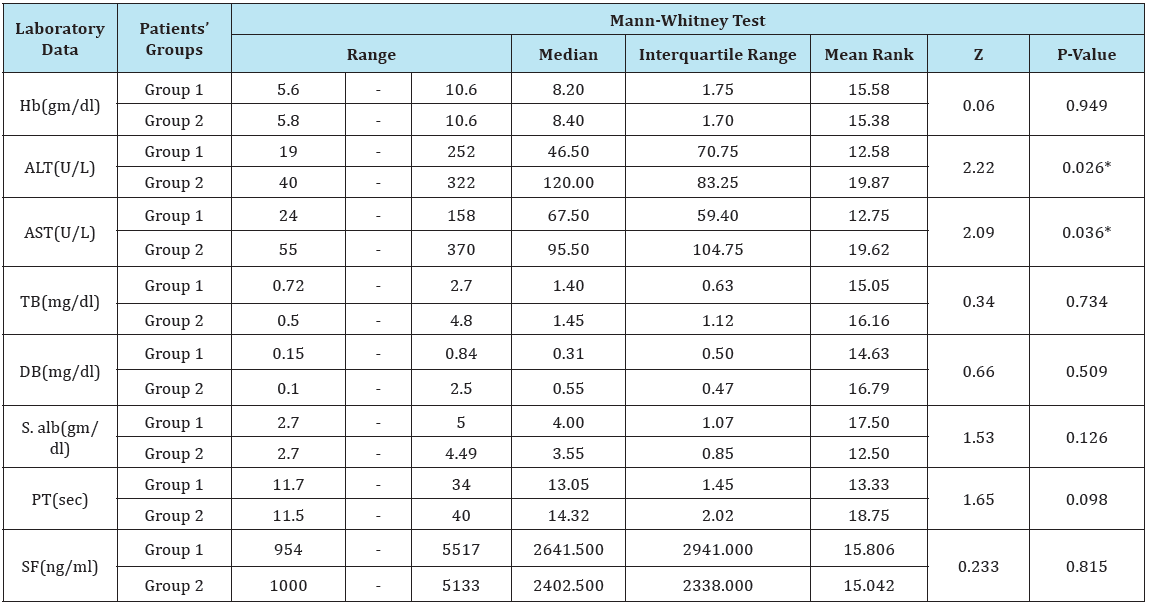
*Hb= Hemoglobin; AST= Aspartate Aminotransferase; ALT= Alanine Aminotransferase; TB= Total Bilirubin; DB= Direct Bilirubin; S. alb= Serum Albumin; PT= Prothrombin Time; SF= Serum Ferritin.
There was a highly significant increase in LIC with associated significant changes in other MRI parameters (significant decrease in T2* and significant increase in R2*) in patients with moderate to severe group (group 2) as compared to those of the mild group (Table 3).
Table 3: MRI findings in patients’ groups before treatment.
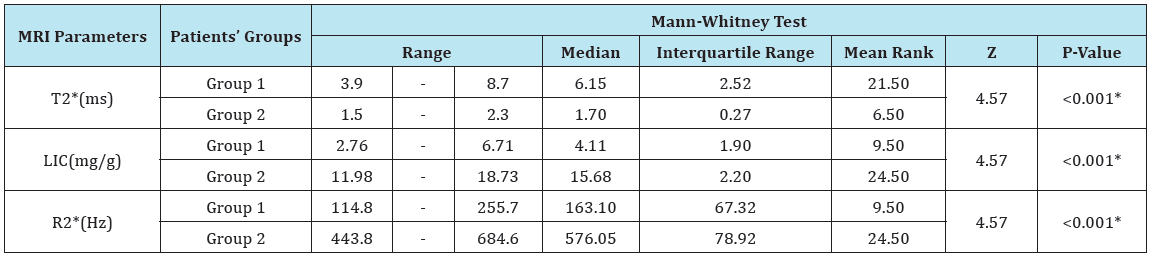
LIC = Liver Iron Concentration; ms = millisecond; Hz = Hertz; T2* represents the echo time necessary for a tissue to become twice as dark. Alternatively, image darkening can be expressed by R2* where it represents the rate of darkening. R2* is directly proportional to iron concentration; R2* values are simply 1000/T2* and vice versa.
Table 4: Correlation between LIC and other MRI findings.
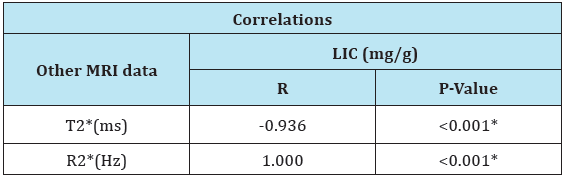
LIC=Liver Iron Concentration; ms = millisecond; Hz = Hertz; T2* represents the echo time necessary for a tissue to become twice as dark. Alternatively, image darkening can be expressed by R2* where it represents the rate of darkening. R2* is directly proportional to iron concentration; R2* values are simply 1000/ T2* and vice versa.
There was a negative correlation between LIC and T2* and positive correlation between LIC and R2* (Table 4). But, there was no correlation between different MRI parameters and SF level (Table 5). There was a significant increase in Hb level after treatment as compared to pretreatment level in mild group and significant decrease in serum ferritin level after treatment as compared to pretreatment level in moderate to severe group. But no significant differences in total bilirubin, direct bilirubin, serum albumin and prothrombin time in both groups (Table 6).
Table 5: Correlation between MRI finding and serum ferritin.
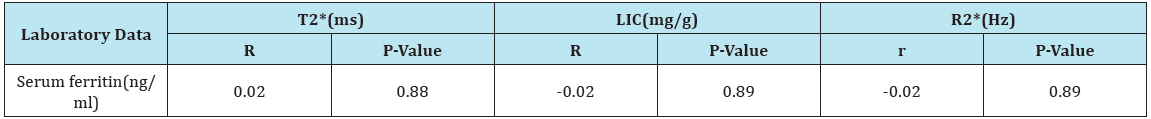
LIC= Liver Iron Concentration; ms = millisecond; Hz = Hertz; T2* represents the echo time necessary for a tissue to become twice as dark. Alternatively, image darkening can be expressed by R2* where it represents the rate of darkening. R2* is directly proportional to iron concentration; R2* values are simply 1000/T2* and vice versa.
Table 6: Laboratory findings in patients’ groups before and after spirulina therapy.
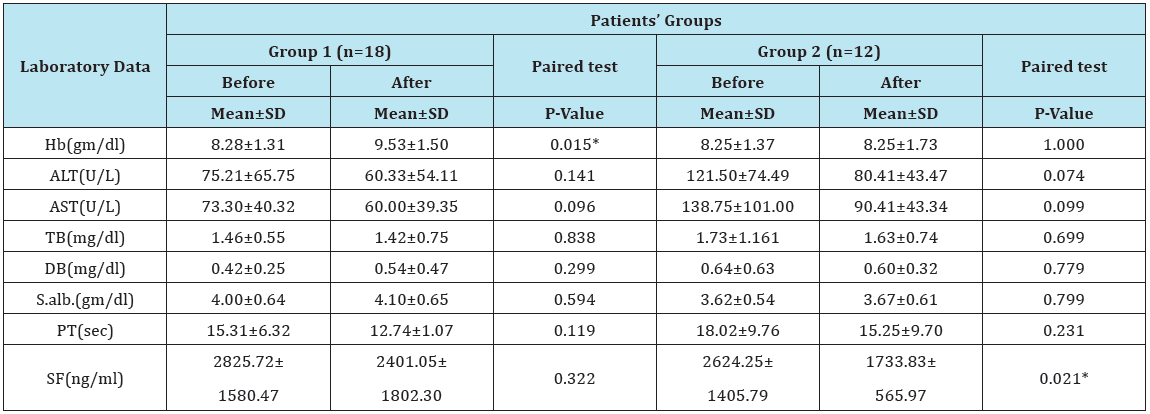
Hb= Hemoglobin; AST= Aspartate Aminotransferase; ALT= Alanine Aminotransferase; TB= Total Bilirubin; DB= Direct Bilirubin; S. alb.= Serum Albumin; PT= Prothrombin Time; SF= Serum Ferritin.
There were no significant changes in the values of different MRI parameters before and after Spirulina therapy in the group of mild liver iron overload. However, there was a highly significant decrease in values of LIC with associated significant changes in other MRI parameters (significant increase in T2* and significant decrease in R2*) after treatment as compared to their levels before treatment in patients of the moderate to severe group (Table 7 & 8, Figure 1A & 1B, Figure 2A & 2B ).
Figure 1: Hepatic iron overload by MRI in child with thalassemia before spirulina therapy.
A: Gradient T2* image reveal decreased liver signal intensity.
B: Spread sheet before spirulina therapy reveal severe hepatic iron overload (liver iron concentration =16.8mg/g).
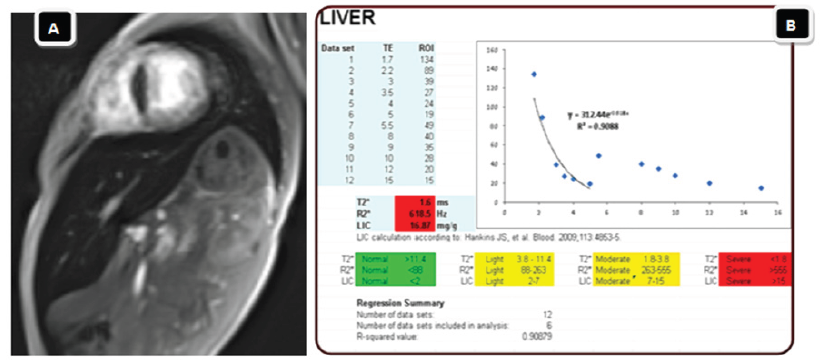
Figure 2: Hepatic iron overload by MRI in child of thalassemia after spirulina therapy.
A: Gradient T2* image reveal decreased liver signal intensity.
B: Spread sheet after spirulina therapy reveal regressive course with mild hepatic iron overload (liver iron concentration = 3.7mg/g).

Table 7: MRI findings in thalassemic patients’ groups before and after Spirulina therapy.
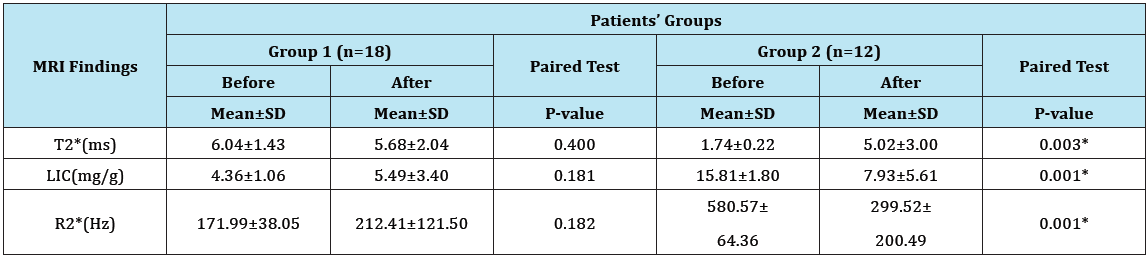
LIC= Liver Iron Concentration; ms = millisecond; Hz = Hertz; T2* represents the echo time necessary for a tissue to become twice as dark. Alternatively, image darkening can be expressed by R2* where it represents the rate of darkening. R2* is directly proportional to iron concentration; R2* values are simply 1000/T2* and vice versa.
Table 8: Frequency of cases with different severity of LIC before and after treatment among the improved and non-improved patients.
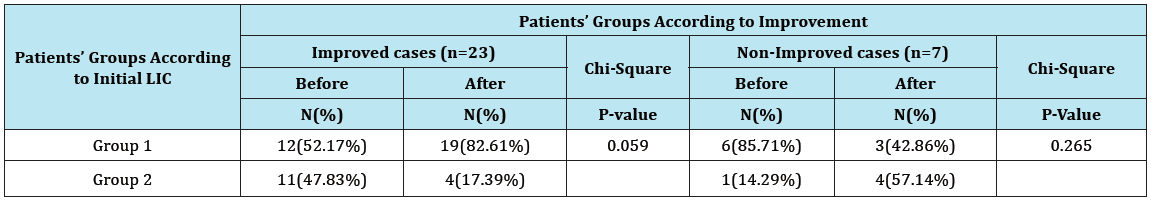
*LIC = liver iron concentration.
Discussion
To the author’s best knowledge, this study is the first clinical trial to evaluate the effect of Spirulina supplementation on LIC in patients with thalassemia as assessed by MRI. The present study showed that there were no significant differences between patients’ groups as regard to age, sex, disease duration, and anthropometric measurements. This can be explained by matching comparison of both groups as the same inclusion and exclusion criteria were applied to patients of both groups. Also, there were no significant differences between patients’ groups as regard to the frequency of blood transfusion.
On clinical examination of patients, there were no significant differences among patients of both groups as regard to pallor, jaundice, hepatomegaly, splenomegaly and splenectomy as they were suffering from the same hemolytic disease. However, the percentage of patients who had splenectomy was insignificantly higher among patients of mild group. In agreement with this finding, Angelucci et al. [23] reported that splenectomy and its timing had a little insignificant effect on LIC and total body iron(108). In this study, the mean values of ALT and AST were significantly higher in patients with moderate to severe group compared to patients of mild group. This finding was in agreement with thatobtained by Anwar et al. [24]. It could be due to the role of higher LIC combined with HCV infection in exacerbation of inflammation and progression of liver fibrosis resulting in increased aminotransferase activity.
As regards to the mean values of SF was insignificantly higher among patients of mild group. This finding is in harmony with Hoffbrand et al. [25] who reported that a single measurement of SF alone is a poor indicator of iron burden in the transfusiondependent patient, particularly if the patient also has HCV infection as the situation in our study.
This study showed that there was a significant increase in LIC associated with significant changes in other MRI parameters (significantly decreased T2* and significantly increased R2*) in patients with moderate to severe group as compared to those of the mild group. This result was in agreement with that described by Alústiza et al. [26] who reported that the larger the liver iron overload, the greater the decrease of signal intensity (SI) on MR images which is represented with T2* as liver parenchyma darkens progressively with increased echo time. These results can be explained by the paramagnetic properties of iron that can affect susceptibility of tissue and produce changes in the magnetic field so that high iron overload lead to decrease in relaxation times (T2*,T2) and increase in relaxation rates (R2,R2*) [27]. Another explanation of this finding could be attributed to the mechanism of MRI, which does not image the iron directly, but instead images water protons as they diffuse near iron deposits in the tissue of interest such as heart and liver. The iron acts as little magnets, destroying the homogeneity of the magnetic field in iron laden tissues. The moving water protons each experience significantly different magnetic profile and become desynchronized from one another. This causes the image to darken at a rate proportional to the iron concentration [27]. Another possible mechanism includes the presence of tissue iron that introduces microscopic B0 field inhomogeneities that result in rapid signal dephasing and increased rate of R2* relaxation in gradient-echo images [28]. Also, relaxation occurs through proton chemical exchange between bulk water and exchangeable protons bound to iron-containing proteins. Iron electrons enhance the relaxation of the protein-bound water protons. Through chemical exchange, enhanced relaxation of bound water protons is then transferred to the bulk water protons, leading to an R2 increase of bulk water [29]. Regardless of the specific mechanism, it has been shown that R2 increases monotonically with LIC [30].
The increased iron stores may be due to repeat blood transfusion, released from damaged hepatocytes, to virus infection itself or to genetic determinants so that the iron can influence the progression of chronic hepatitis C [31,32]. Patients with chronic hepatitis C have mildly to moderately increased hepatic iron concentration and occasionally have severe hepatic iron overload [33]. In this study, there was a negative correlation between LIC and T2* and positive correlation between LIC and R2*. These findings agreed with other authors [34,35] who reported that LIC is greatly associated with T2* and R2*. It was reported that T2* is a magnetic relaxation property of any tissue and is inversely related to intracellular iron stores [36]. This study showed that there was no correlation between different MRI parameters and SF level. This finding was in agreement with that reported by other authors [37,38]. Also, Azarkeivan et al. [39] reported that the correlation between SF and liver T2* greatly weakened in patients with SF readings higher than 4000mg/ml. Furthermore, Neufeld [40] reported that SFasa surrogate measure of LIC is highly varied Likewise, Angulo et al. [41] confirmed that there was no correlation between SF and LIC assessed by hepatic MRI in patients with thalassemia.
Furthermore, Sher et al. [42] confirmed that attempts to correlate SF with LIC have failed to demonstrate a linear relationship between the two parameters and discrepancies have frequently been observed. Likewise, in a previous study grouping patients with mild to moderate siderosis and those with severe and very severe siderosis, statistically non-significant difference was observed between SF levels of the two groups and no significant correlation was seen with ferritin levels when different severities of siderosis were compared, although a trend toward significance was evident when the ferritin values of patients with less severe iron load were compared with those of greater iron overload [43]. The grade of iron load is determined by visual evaluation and a standardized definition is difficult because it depends on the thickness of the intracellular iron granules. On the other hand, the distribution of the granules and whether they involve both Kupffer’s cells and hepatocytes can easily be seen. As hepatic fibrosis progresses and inflammatory cells decrease the liver gradually loses its capacity to synthesize and release ferritin, and in the final stage of cirrhosis, the ferritin level is generally low [43]. The poor correlation between SF and LIC in patients with β-thalassemia could be explained by many factors. Serum ferritin can be affected by the presence of hepatitis C which increases SF and vitamin C which determine allocation of iron between macrophages and parenchymal sites [32]. Another explanation of this finding is that SF which is an acute phase reactant generated in response to inflammation, contains much less iron than normal ferritin and this is very apparent in patients with β-thalassemia and hepatitis C virus [37] Furthermore, previous studies indicated that SF in contrast to tissue ferritin has low iron content even in iron loaded patients [44]. In contrast to previous findings, many studies showed different correlations between MRI findings and SF. Alexopoulou et al. [45] reported that R2* had significant positive correlation with SF. Across sectional study by Piga et al. [46] showed positive correlation between LIC and SF. Similarly, other authors [35,47,48] had reported significant negative correlation between SF and T2*. The causes of discrepancies between these studies could be related to differences in the number or ethnicity or disease duration of patients enrolled in various studies, or to differences in methods of measurements of LIC or SF used in these studies.
In the present study, there was significant decrease in SF in HCV-infected patients with thalassemia after spirulina therapy as compared to pretreatment levels. This finding is consistent with many authors [14, 49,50]. This can be explained by the hepatoprotective and chelating effects of spirulina therapy with subsequent decreased in LIC. Spirulina therapy has combined antioxidant effects and chelating activity that can produce upregulation of antioxidant enzymes as well as excretion of iron from the body by effective chelation. Another suggested mechanism for reduction of SF after Spirulina therapy is the high content of metallothioneins in Spirulina which are proteins capable of binding heavy metals and excreting them from the body [15,51].
The mean values of LIC was significantly lower (with associated significant changes in the mean values of T2* and R2*) in patients of moderate to severe group after spirulina therapy compared to their pretreatment values. These results could be explained by the hepatoprotective and chelating effects of spirulina therapy with subsequent decreased LIC. The highly significant decrease in the values of SF and values of LIC (with associated highly significant changes in T2* and R2*) after spirulina therapy as compared to their levels before treatment in patients of the moderate to severe group but not in the group of mild liver iron overload could be explained by the great Spirulina’s chelating and hepatoprotective effects and the previous findings of other studies showing that all chelating agents are highly effective in their action to decrease LIC and SF levels in patients with the highest grades of liver siderosis and elevated SF levels. Several previous studies [52-55] reported that the use of different chelating agents in thalassemic patients produced significant decrease in SF levels in patients with baseline values above 2500μg/L but not with values below 2,500μg/L. Based on the results of these previous studies and results of our study, we could assume that the use of Spirulina in our patients produced significant reduction in the mean values of LIC and marked improvement in patients with the highest degrees of LIC compared to patients with mild LIC before spirulina therapy.
A key limitation of this research is: 1-The lack of a control group of children with thalassemia without HCV infection 2-The lack of control HCV-infected thalassemic children without Spirulina treatment. 3- The small sample size and the shorter duration of the study. Therefore, many randomized controlled clinical trials with large sample size and longer durations of Spirulina therapy should be conducted for further evaluation of the promising Spirulina’ effects on LIC and other parameters in children with thalassemia with and without HCV infection.
Conclusion
From this study, we can conclude that, spirulina therapy may have favorable effects on lowering the values of LIC in children with β-Thalassemia infected with HCV.
References
- Mohammad I, Al Doski FS (2012) Assessment of liver functions in thalassemia. Tikrit Journal of Pharmaceutical Sciences 8(1): 87-95.
- Youssef SM, El Alfy MS, Osman AL, Dina AK, Mervat AF, et al. (2012) Rapid detection of multiple β-globin gene mutations by a real-time polymerase chain reaction in β-thalassemia carriers. Egypt Jornal of Hematol 37(3): 147-155.
- Di Marco V, Capra M, Gagliardotto F, Borsellino Z, Cabibi D, et al. (2008) Liver disease in chelated transfusion-dependent thalassemics: the role of iron overload and chronic hepatitis C. Hematologica 93(8): 1243- 1246.
- Elalfy MS, Esmat G, Matter RM, Abdel Aziz HE, Massoud WA (2013) Liver fibrosis in young Egyptian beta-thalassemia major patients: relation to hepatitis C virus and compliance with chelation. Ann Hepatol 12(1): 54- 61.
- Malik S, Syed S, Ahmed N (2009) Complications in transfusiondependent patients of b-thalassemia major: a review. Pakistan Jornal of Medical Science 25(4): 678-682.
- Manning DS, Afdhal NH (2008) Diagnosis and quantitation of fibrosis. Gastroenterology 134(6): 1670-1681.
- Xu HG, Fang JP, Huang SL, Li HG, Zhong FY (2003) Diagnostic values of serum levels of HA, PC III, CIV and LN to the liver fibrosis in children with beta-thalassemia major. Zhonghua Er Ke Za Zhi 41 (8): 603-606.
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, et al. (2006) Diagnosis of cirrhosis by transientelastography (FibroScan): a prospective study. Gut 55(3): 403-408.
- Rockey DC, Bissell DM (2006) Noninvasive measures of liver fibrosis. Hepatology 43(2 suppl 1): 113-120.
- De Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, et al. (2006) Diagnosis of hepatic fibrosis andcirrhosis bytransientelastographyinHIV/ hepatitisC virus-coinfectedpatients. J Acquir Immune Defic Syndr 41(2): 175-179.
- Di Marco V, Bronte F, Cabibi D, Calvaruso V, Alaimo G, et al. (2010) Noninvasive assessment of liver fibrosis in thalassaemia major patients by transient (TE)-lack of interference by irondep63osition. Br J Haematol 148(3): 476-479.
- Khan Z, Bhadouria P, Bisen PS (2005) Nutritional and therapeutic potential of Spirulina. Curr Pharm iotechnol 6(5): 373-379.
- Blinkova LP, Gorobets OB, Baturo AP (2001) Biological activity of Spirulina. Zh Mikrobiol Epidemiol Immunobiol 2: 114-118.
- Ferreira Hermosillo A, Torres Duran PV, Juarez Oropeza MA (2010) Hepatoprotective effects of Spirulina maxima in patients with nonalcoholic fatty liver disease: a case series. J Med Case Rep 4: 103.
- Gad AS, Khadrawy YA, El Nekeety AA, Mohamed SR, Hassan NS, et al. (2011) Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition 27(5): 582-589.
- Bermejo P, Piñero E, Villar AM (2008) Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chemistry 110(2): 436-445.
- Grzanna R, Polotsky A, Phan PV, Pugh N, Pasco D, et al. (2006) Immolina, a high-molecular-weight polysaccharide fraction of Spirulina enhances chemokine expression in human monocytic THP-1 cells. J Altern Complement Med 12(5): 429-435.
- Mazo VK, Gmoshinskñ IV, Zilova IS (2004) Microalgae Spirulina in human nutrition. Vopr Pitan 73(1): 45-53.
- Abdel Wahhab MA, Ahmed HH, Hagazi MM (2006) Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J Appl Toxicol 26(3): 229-238.
- Ray S, Roy K, Sengupta C (2007) In vitro evaluation of protective effects of ascorbic acid and water extract of Spirulina plantesis (blue green algae) on 5-fluorouracil-induced lipid peroxidation. Acta Pol Pharm 64(4): 335-344.
- Fernandes JL, Sampaio EF, Verissimo MP, Verissimo M, Pereira FB, et al. (2011) Heart and liver T2* assessment for iron overload using different software programs. Eur Radiol 21(12): 2503-2510.
- Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, et al. (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113(20): 4853-4855.
- Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, et al. (2000) Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med 343(5): 327-331.
- Anwar M, Wood J, Manwani D, Benjamin T, Suzette OO, et al. (2013) Hepatic Iron Quantification on 3 Tesla (3T) Magnetic Resonance (MR): Technical Challenges and Solutions. Radiol Res Pract 2013.
- Hoffbrand AV, Cohen A, Hershko C (2003) Role of deferiprone in chelation therapy for transfusional iron overload. Blood 102: 17-24.
- Alústiza JM, Artetxe J, Castiella A, Cristina A, José IE, et al. (2004) MR quantification of hepatic iron concentration. Radiology 230(2): 479-484.
- Wood JC, Ghugre NR (2008) MRI assessment of excess iron in thalassemia. Sickle cell disease and other iron overload diseases. Hemoglobin 32(1- 2): 85-96.
- Ghugre NR, Wood JC (2011) Relaxivity-iron calibration in hepatic iron overload: probing underlying biophysical mechanisms using a Monte Carlo model. Magn Reson Med 65(3): 837-847.
- Gossuin Y, Muller RN, Gillis P (2004) Relaxation induced by ferritin: a better understanding for an improved MRI iron quantification. NMR Biomed 17(7): 427-432.
- St Pierre TG, Clark PR, Chua anusorn W, Fleming AJ, Jeffrey GP, et al. (2005) Non invasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105(2): 855-861.
- Sartori M, Andorno S, La Terra G, Boldorini R, Leone F, et al. (1998) Evaluation of iron status in patients with chronic hepatitis C. Ital J Gastroenterol Hepatol 30(4): 396-401.
- Prabhu R, Prabhu V, Prabhu RS (2009) Iron overload in Beta Thalassemia- A Review. J Biosci Tech 1(1): 20-31.
- Riggio O, Montagnese F, Fiore P, Folino S, Giambartolomei S, et al. (1997) Iron overload in patients with chronic viral hepatitis: how common is it? Am J Gastroenterol 92(8): 1298-301.
- Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, et al. (2005) MRI R2 and R2+ mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106(4): 1460-1465.
- Fahmy HS, Khater NH, Elshahat HM, Ahmed AM, El Hadidyd SS, et al. (2015) Reassessing the value of MRI T2* in evaluation of hepatic and myocardial iron concentration: An institutional study. The Egyptian journal of radiology and nuclear medicine 46(4): 1085-1090.
- Anderson LJ (2011) Assessment of iron overload with T2* magnetic resonance imaging. Prog Cardiovasc Dis 54(3): 287-294.
- Nielsen P, Gunther U, Durken M, Fischer R, Düllmann J (2000) Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. J Lab Clin Med 135(5): 413-418.
- Argyropoulou MI, Kiortsis DN, Astrakas L, Metafratzi Z, Chalissos N, et al. (2007) Liver, bone marrow, pancreas and pituitary gland iron overload in young and adult thalassemic patients: a T2 relaxometry study. Eur Radiol 17(12): 3025-3030.
- Azarkeivan A, Hashemieh M, Akhlaghpoor S, Shirkavand A, Yaseri M, et al. (2013) Relation between serum ferritin and liver and heart MRI T2* in beta-thalassemia major patients. East Mediterr Health J 19(8): 727- 732.
- Neufeld EJ (2006) Oral chelators, deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood 107(9): 3436-3441.
- Angulo IL, Carneiro DT, Baffa O, Antonio AC, Jorge E, et al. (2008) Determination of iron-overload in thalassemia by hepatic MRI and ferritin. Rev Bras Hematol Hemoter 30(6): 449-452.
- Sher GD, Beluzzo N, Milone SD (1994) Blood 1: 1437-1450.
- Mazza P, Giua R, Marco SD, Bonetti MG, Amurri B, et al. (1995) Iron overload in thalassemia: Comparative Analysis of Magnetic Resonance Imaging, serum ferritin and iron content of the liver. Hematologica 80(5): 398-404.
- Halliday JW, Ramm GA, Powell LW (1994) Cellular iron processing and storage: the role of ferritin. In: Brock JH, Halliday JW, Pippard MJ, Powell LW (Eds.), Iron metabolism in health and disease. WB Saunders, USA, pp. 121-127.
- Alexopoulou E, Stripeli F, Baras P, Seimenis I, Kattamis A, et al. (2006) R2 relaxometry with MRI for the quantification of tissue iron overload in beta-thalassemic patients. J Magn Reson Imaging 23(2): 163-170.
- Piga A, Longo F, Musallam KM, Cappellini MD, Forni GL, et al. (2013) Assessment and management of iron overload in β-thalassaemia major patients during the 21st century: a real-life experience from the Italian WEBTHAL project. Br J Hematol 161(6): 872-883.
- Fischer R, Longo F, Nielsen P, Engelhardt R, Hider RC, et al. (2003) Monitoring long-term efficacy of iron chelation therapy by deferiprone and desferrioxamine in patients with β-thalassaemia major: application of SQUID biomagnetic liver susceptometry. Br J Hematol 121(6): 938- 948.
- Zamani F, Razmjou S, Akhlaghpoor S, Eslami SM, Azarkeivan A, et al. (2011) T2* magnetic resonance of liver in thalassemic patients in Iran. World Journal of Gastroenterology 17(4): 522-525.
- Yakoot M, Salem A (2012) Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. A pilot randomized, comparative clinical trial. BMC Gastroenterology 12:32.
- Mazokopakis EE, Papadomanolaki MG, Fousteris AA, Kotsiris DA, Lampadakis IM, et al. (2014) The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: a prospective pilot study. Ann Gastroenterol 27(4): 387-394.
- Bermejo P, Piñero E, Villar AM (2008) Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chemistry 110(2): 436-445.
- Al Refaie FN, Wonke B, Hoffbrand AV, Wickens DG, Nortey P, et al. (1992) Efficacy and possible adverse effects of the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-1 (L1) in thalassemia major. Blood 80(3): 593-599.
- Agarwal MB, Gupte SS, Viswanathan C, Vasandani D, Ramanathan J, et al. (1992) Long-term assessment of efficacy and safety of L1, an oral iron chelator, in transfusion-dependent thalassemia: Indian trial. Br J Hematol 82(2): 460-466.
- Olivieri NF, Brittenham GM, Matsui D, Matitiahu B, Laurence MB, et al. (1995) Iron chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med 332: 918-922.
- Cohen AR, Galanello R, Piga A (2000) Long-term safety and efficacy of the oral iron chelator deferiprone. Blood 96: 443-452.
© 2017 El Shanshory MR, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






