- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Photocatalytic Activity of Methylene Blue Using Zinc Nanoparticles Synthesized from Gymnema sylvestre And Antimicrobial Assay
Murali Santhosh Kumar, N Suprajaa and E David*
Department of Biotechnology, Tamil Nadu, India
*Corresponding author: E David, Department of Biotechnology, Serkkadu, Vellore-632001, Tamil Nadu, India
Submission: February 08, 2019;Published: February 25, 2019

ISSN 2640-9275 Volume2 Issue4
Abstract
Nanoparticles of the Zn were synthesized by Gymnema sylvestre leaf extract characterized by UV (UV-visible spectrum), FT-IR (Fourier transformance infra-red spectroscopy), X-ray diffraction (XRD), DLS (Dynamic light scattering analysis) and scanning electron microscopy (SEM). The XRD patterns of the Zn and nanoparticles could be indexed to hexagonal and Wurtzite phase, respectively. Aggregated nanoparticles of Zn with spherical-like shapes were observed with particle diameter in the range of 200-400μm. These nanoparticles were used for photocatalytic degradation of methylene blue dye under solar light irradiation. The ZnNPs shown very good antimicrobial Assay towards microbes isolated from contaminated dye soil..
Keywords: Gymnema sylvestre; ZnNPs; photocatalytic activity; Dye degradation; Antimicrobial Assay
Abbreviations: UV: UV- Visible Spectrum; FT-IR: Fourier Transformance Infra-Red Spectroscopy; XRD: X-Ray Diffraction; DLS: Dynamic Light Scattering Analysis; SEM: Scanning Electron Microscopy
Introduction
In recent years, the contamination of surface and ground water has increased due to population growth. The main sources of environmental contamination are organic dyes used in the food and textile industries due to their high toxicity and their nonbiodegradability, which have carcinogenic effects on humans. MB dye is used by different industries, for example, as a dye in silk; as a food coloring additive; and as a dye in wool, leather, cotton, jute, and paper [1]. Methylene blue dyes have strong effects on the immune and reproductive systems and exhibit potential carcinogenic and genotoxic effects. Thus, these hazardous dyes must be removed from industrial effluents. Many methods, such as biological treatment, adsorption, and photo catalysis [2], have been used for removal of these dyes from industrial effluents. The use of photo catalysts to degrade organic compounds in contaminated air or water or to convert them into harmless chemicals has been extensively studied to decrease the damage caused by organic dye pollution to the environment and humans [3]. Therefore, heterogeneous photocatalysis is an interesting area of research because the method allows for complete mineralization of these environmentally hazardous dyes Zinc Oxide (Zn) belongs to a category of n-type semiconductors having wide energy band gap of 3.37eV. Recently, Zn has attracted much attention as a promising photocatalytic material for removal of organic pollutants, which present in wastewater, all because of its high catalytic activity, moderate preparation cost and environmentally benign nature [4]. Nanoscale Zn particles possess significant surface area and large number of active sites ensure increased surface catalyzed reaction rates thus promoting photocatalysis [5]. However, the large energy band gap of Zn permits electronic excitations only with photons having energy below 400nm (UV spectrum). High degree of recombination of photogenerated species is another limitation associated with Zn which is responsible for low photocatalytic activity [6,7]. These shortfalls can be remediated by modifying Zn such as to extend its absorption threshold to the visible spectrum and limiting the rate of electron/hole pair recombination. Different attempts were achieved recently to improve the activity of Zn photocatalyst.
Nanotechnology deals with the synthesis of nanoparticles with controlled size, shape, and dispersity of materials at the nanometer scale length and their potential use for human wellbeing. Nanometer sized materials have a high surface area; and a high fraction of surface atoms have been studied because of their exclusive properties such as optic, electronic, and catalytic Among all nanoparticles noble metal nanoparticles have enormous applications in diverse areas such as bioimaging, sensor, diagnosis, and novel therapeutic in biomedical field. Metallic silver and silver nanoparticles were recently applied as antimicrobial agents in various products such as cosmetics, animal feed coating of catheters, wound dressing and water purification with a minimal risk of toxicity in humans. Now a days the biological systems were eagerly used for nanoscale material synthesis and assembly is an alternative method of physical and chemical process. Green approach of nanoparticles synthesis by biological entities has been gaining great advantages which are environmental benign, less toxic, and time consuming and, it is a single step process. Currently, plant and plant derived materials are used for nanoparticles synthesis which is more compatible than the microbe-mediated nanoparticles synthesis process because they eliminate the culture maintenance and are easy to handle. Nanoparticles synthesis by medicinal plants shows more benefit; they may enhance the antibacterial activity of silver nanoparticles, because the medicinally valuable active biomolecule present in the plants may bind on the surface of the nanoparticles and reduce the silver ions to silver nanoparticles [8]. Nanoparticles can be organized into four types namely carbon based, metal based, dendrimers and composites nanoparticles are also referred to as organic nanoparticles. Spherical and ellipsoidal carbon nanoparticles are called as fullerenes, while cylindrical ones are called nanotubes. Quantum dots, nanogold, Nano silver and metal oxides (titanium oxide) are examples of metal-based nanoparticles. Dendrimers are Nano sized polymers which can be synthesized to perform specific chemical functions. Composites are c nanoparticles with other nanoparticles or with larger sized and heavier compounds. Silver nanoparticles are most commonly used in water filters and biosensors [9,10].
Gymnema sylvestre is a perennial woody vine that grows in tropical areas of India, Africa, and Australia and has been used for medicinal purposes in Ayurvedic medicine. Common names include gymnema, Australian cow plant, and Periploca of the woods, and the Hindi term gurmar which means “sugar destroyer”. The leaves and extracts contain Gymnema acids, the major bioactive constituents that interact with taste receptors on the tongue to temporarily suppress the taste of sweetness [11]. Dyes are defined as colored, ionizing and aromatic organic compounds which show affinity towards the substrate to which it is being applied and they are extensively utilized in the textile industry [12]. These nonbiodegradable substances must be removed from the environment and pose to be a dire environmental crisis. Many methods are regularly used for reducing dyes like activated carbon sorption, flocculation, electro coagulation, UV-light degradation and redox treatments [13]. However, due to the ineptitude of these methods in one way or the other, the present situation necessitates bet and improved removal methods. Lately, studies have found that Zn nano particles are good, highly efficient and stable catalysts under ambient temperature with visible light illumination for degrading organic compounds and dyes Hence, the purpose of the present study was to assess the degrading property of the synthesized Zn nanoparticles from Gymnema sylvestre leaves extract towards methylene blue and Antimicrobial Assay.
Materials and Methods
Collection of leaves and preparation of ZnNPs
Leaves of Gymnema sylvestre were collected from the Acharya N G Ranga Agricultural University, Tirupati, Andhra Pradesh, India. 5g wet weight of fresh leaves was cut into fine pieces and washed with distilled water and boiled with 100mL of double distilled water for 15 min at 70 °C. Boiled mixture was filtered through Whatman No. 1 filter paper and collects the supernatant of leaf extract to that 90ml of zinc nitrate solution is added left for overnight when there is a color changes from color less to pale yellow color indicate the formation of ZnNPs and remaining leaf solution stored at 4 °C for further studies.
Collection of dye sample
The Dye sample were collected from Tirupur industry from Tirupur District in Tamilnadu, India. These dye samples were sended to Biotechnology department, Thiruvalluvar University, Vellore and the microbes were collected from dye soil samples through serial dilution and pure microbes were isolated by using streaking method in nutrient agar medium for bacteria and potato dextrose agar for fungi.
Characterization of zinc nanoparticles
The UV-Vis spectrum of this solution was recorded in spectra 50 Analytikjena Spectrophotometer, which was operated in the wavelength range of 400-800 nm. The FT-IR spectrum was taken in the mid IR region of 400-4000cm-1. The spectrum was recorded using attenuated total reflectance technique. The crystalline structure of the nanoparticles was determined using the XRD technique. The XRD pattern was recorded using computer controlled XRD-system, JEOL, and Model: JPX-8030 with Cu Kα radiation (Ni filtered=13,418A°) at the range of 40kV, 20A. The aqueous suspension of the synthesized nanoparticles was filtered through a 0.22lm syringe driven filter unit, and the hydro dynamic diameter (HDD) of the distributed nanoparticles was measured by the principle of dynamic light scattering (DLS) using Nano partica (HORIBA, SZ-100) compact scattering spectrometer, the surface morphological studies of the ZnNPs samples were carried out with scanning electron microscope (SEM) (Hitachi’s SU6600) at magnification ranging from 10 to 600,0000 operated at an accelerating voltage of 30kV.
Photocatalytic degradation of dye
Typically, 10mg of methylene blue dye was added to 1000mL of double distilled water used as stock solution. About 10mg of biosynthesized zinc nanoparticles was added to 100mL of methylene blue dye solution. A control was also maintained without addition of zinc nanoparticles. Before exposing to irradiation, the reaction suspension was well mixed by being magnetically stirred for 40min to clearly make the equilibrium of the working solution. Afterwards, the dispersion was put under the sunlight and monitored from morning to evening sunset. At specific time intervals, aliquots of 5mL suspension were filtered and used to evaluate the photocatalytic degradation of dye. The absorbance spectrum of the supernatant was subsequently measured using UVVis spectrophotometer at the different wavelength. Concentration of dye during degradation was calculated by the absorbance value at 650nm [14].
Antimicrobial activity of plant biosynthesized zinc nanoparticles
The antimicrobial activity of Gymnema sylvestre leaf extract zinc nanoparticles was examined based on colony formation by In-vitro Petri dish assays (disc diffusion). Each fungal and bacterial isolate was cultured on growth media that induced prolific conidia and bacterial production. The fungus isolates were grown on potato dextrose agar medium, and bacterial isolates were grown on nutrient agar medium. Conidia were collected from cultures that were incubated at 37 °C for 10 days (fungi), and bacterial cultures were collected from cultures that were incubated at 37 °C for 2 days for (bacteria) and diluted with sterile, deionized water to a concentration of 106spores ml-1. Aliquots of the conidial suspension and bacterial suspension were mixed with serial concentrations of zinc preparations to a final volume of 1ml and were also mixed with sterile, deionized water as control. A 10μl subsample of the conidia and Gymnema sylvestre zinc mixture stock was taken at 50± 0.8, 100± 1.1 and 170± 1.4 ppm after zinc treatments and diluted 100-foldwith the deionized water. A 10μl aliquot of the diluted spore suspension was spread on PDA (Becton, Dickson and Company, Sparks, MD) medium. Three PDA plates for fungi and three NA plates for bacteria per each combination of exposure zinc concentration were tested. The filter paper disc dipped in different ppm and inserted on mediums (PDA), and then, the plates were incubated at 37 °C for 5days for fungi and 3days for bacteria, respectively. The average number of colonies from zinc-treated spore suspensions (fungi) and (bacteria) was compared with the number on the water control (percent colony formation). The zone size was determined by measuring the diameter of the zone in mm [15].
Statistical analysis
All the data from three independent replicate trials were subjected to analysis using Statistical package for the Social Sciences (SPSS) version 16.0. The data are reported as the mean ± SD.
Results and Discussion
Figure 1:Gymnema sylvestre leaves.

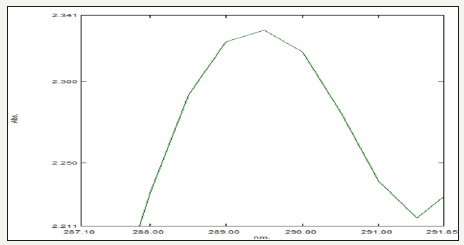
UV-visible spectroscopy characterization method was then used to monitor formation of ZnNPs synthesized from Gymnema sylvestre (Figure 1) and its properties. The absorption of light by the nanoparticles at different wavelengths provides an indication of particle size, while the breadth of the peaks signifies the particle size distribution. The characteristic SPR band of ZnNPs is in the range of 289nm [16] (Figure 2).
Figure 2:UV-Visible spectrum of Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
The FT-IR spectra of Gymnema sylvestre leaf extract mediated ZnNPs. From the FT-IR spectra, as seen in (Figure 3), a series of absorption peaks from 500 to 4000cm-1 are found, corresponding to the carboxylate and hydroxyl impurities in materials. A broad band at around 3747cm-1 is assigned to the OH stretching mode of hydroxyl group. The peaks observed at 3419 cm-1 are due to the asymmetrical and symmetrical stretching of the zinc carboxylate, respectively. 985 and 903cm-1 the content of the carboxylate (COO-) and hydroxyl (-OH) groups in the samples decreased. The carboxylate probably comes from reactive carbon containing species during synthesis and the hydroxyl group results from the hygroscopic nature of Zn [17].
XRD of Zn indicates the well-defined hexagonal shape, resulted by calcining the Gymnema sylvestre based Zn. The peaks observed in the diffractogram in (Figure 4) are assigned in terms of the Miller indices (hkl) assuming a hexagonal crystal structure of Zn, Thus, various well-defined dominant diffraction reflections are seen in the observed XRD pattern which are all related and well matched with the diffraction reflections of hexagonal phase Zn. Average crystallite size as measured from XRD using the Debye-Scherrer formula is 80 nm [14]. These D values refer to average values calculated by Δ2θ1/2 in the (100), (002), (101), (102), (110), (112), and (201) etc prominent peaks of the diffractogram.
Figure 3:FT-IR spectrum of Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
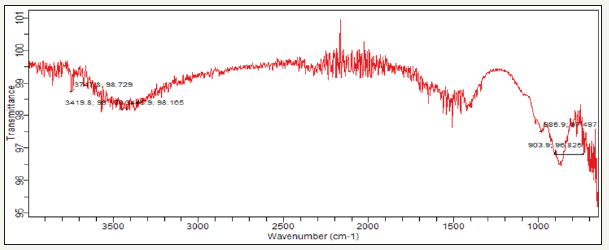
Figure 4:XRD spectrum of Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.

Particle size and zeta potential values were measured using Nano partica SZ-100 (HORIBA). The zeta potential spectra for the Gymnema sylvestre leaf extract nanoparticles were recorded zeta potential verses intensity spectra with zeta potential (mV) on x-axis and intensity (a.u) on y-axis particle size of 100.5 nm and zeta potential of -138.7mV were recorded (Figure 5a,5b), Here the zeta potential value of the zinc nanoparticles indicated good stability with high potential [18].
The SEM micrograph of Zn Nano powder prepared by Gymnema sylvestre leaf extract. A very clean surface is seen by the SEM micrograph with the distinct particles [19]. Particles are nearly spherical with hexagonal flakes, spherical shapes and there is some agglomeration of nanoparticles is seen it is due to the presence of proteins present in the Gymnema sylvestre leaf, so particles are very uniformly distributed with an average particle size of 200-400μm (Figure 6).
Photocatalytic degradation of dye
Photocatalytic degradation of methylene blue was carried out by using green synthesized Zn nanoparticles under solar light.
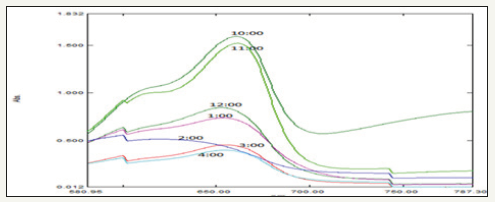
Dye degradation was initially identified by color change. Initially, the color of dye shows deep blue color changed into light blue after the h of incubation with Zn nanoparticles while exposed to solar light (Figure 7). Thereafter light blue was changed into light green. Finally, the degradation process was completed at 7h and was identified by the change of reaction mixture color to colorless. The adsorption of Zn nanoparticles on the methylene blue solution was initially low and further increased with constant increase in time and the percentage of dye degradation. However, at the same time more photo-catalyst would also have induced greater aggregation, which resulted in the decrease in the surface area of the photo-catalyst, making a significant fraction of the catalyst to be inaccessible to adsorbing the dye [20,21]. The experiment was carried out under the following conditions: reaction time of h (10,11,12,1,2,3 and 4) the results show that the photocatalytic activity remained nearly unchanged after five uses, which indicates that the photocatalyst is stable in the photocatalytic oxidation of methylene blue dye solutions. Therefore, this photocatalyst can be separated and recycled while maintaining its stability, making it a promising material for environmental remediation.
Figure 5:DLS spectrum 5a. Particle size 5b. Zeta potential of Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
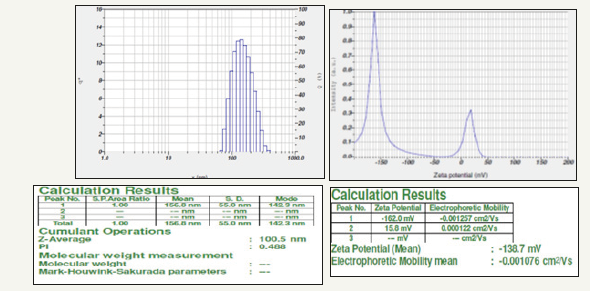
Figure 6:SEM analysis of Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
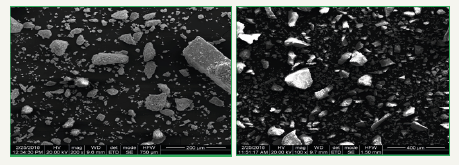
Figure 7:Graph showing UV-visible spectrum of different time intervals of Photo-catalytic degradation of methylene blue using Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
Antimicrobial assay
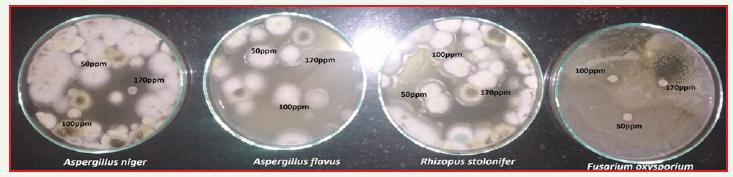
Zn nanoparticles obtained from Gymnema sylvestre leaf extract have shown very strong inhibitory action against fungal Sp, Gram-positive and Gram-negative bacteria (Figures 8 & 9). Three concentrations of ZnNPs (170,100,50ppm) were prepared and were applied against an array of fungal species viz, Aspergillus niger, Rhizopus stolonifer, Aspergillus flavus, Fusarium oxy sporium and bacterial species viz., E. Coli, Bacillus pantothenticus, Pseudomonas fluorescence and Salmonella typhimurium. The higher concentration (170ppm) of leaf mediated synthesized ZnNPs showed significant antimicrobial effect (Tables 1 & 2) compared with other concentrations (100,50ppm) seen in leaf, but when compared to bacterial concentrations fungi shown very good antimicrobial activity towards all concentrations. The inhibitory action of the microbes may be attributed to the loss of replication ability of DNA upon treatment with the Zn ion, besides the fact that expression of ribosomal sub-unit proteins as well as some other cellular proteins and enzymes essential to ATP production becomes inactivated [22]. According to the results, it can be concluded that Zn nanoparticles are effective antibacterial agents both on Gram-positive and Gram-negative bacteria. The same results were confirmed in the study of Supraja et al. [14] in which Gramnegative membrane and Gram-positive membrane disorganization was approved by transmission electron microscopy of bacteria ultrathin sections. To use Zn nanoparticles in in vivo condition, further studies should be performed investigating the toxic effect of Zn nanoparticles on eukaryotic cells.
Table 1:In-vitro antibacterial studies against bacterial pathogens using Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
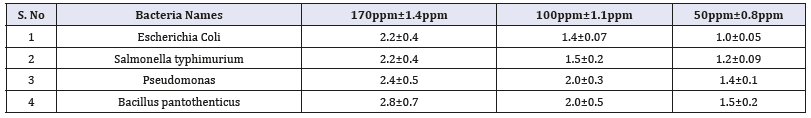
Table 2:In-vitro antifungal studies against fungal pathogens using Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
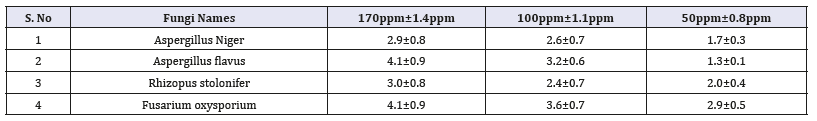
Figure 8:Antibacterial activity of different concentrations (50,100,170ppm) of Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
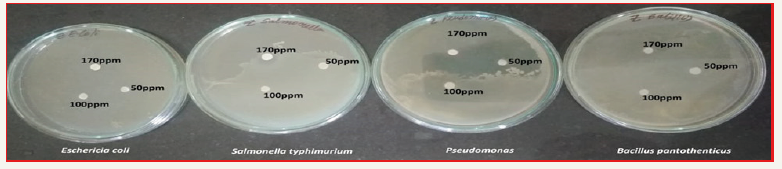
Figure 9:Antifungal activity of different concentrations (50,100,170ppm) of Gymnema sylvestre leaf extract mediated synthesized zinc nanoparticles.
Conclusion
Green synthesis of zinc nanoparticles shows more compatible, ecofriendly, low cost, and less time-consuming process. Herein, the zinc nanoparticles were synthesized by using plant leaf extract of Gymnema sylvestre leaf extract, main absorption peak at 650nm decreased gradually with the extension of the exposure time indicating the photocatalytic degradation of methylene blue dye. The results of photocatalytic studies reveal that the highest photocatalytic activity and stability were obtained for zinc nanoparticle photocatalyst, which can be used to oxidize 94% of methylene blue dye after 7h.
Acknowledgment
Authors are thankful to Acharya N.G. Ranga Agricultural University for providing research facility at institute of Frontier Technology, Regional Agricultural Research Station, Tirupati to carry out this part of the research work.
References
- Cheng W, Wang SG, Lu L, Gong WX, Liu XW, et al. (2008) Interaction between congo red and copper in a binary adsorption system: Spectroscopic and kinetic studies. Biochem Eng J 538-546.
- Pare B, Sarwan B, Jonnalagadda SB (2011) Photocatalytic mineralization study of malachite green on the surface of Mn-doped BiOCl activated by visible light under ambient condition. Appl Surf Sci 258: 247-253.
- Cheng ZJ, Liu TY, Chen XY, Gan HX, Zhang FW, et al. (2012) Study on the electronic structures of the reduced anatase TiO2 by the first-principle calculation. Journal of Physics and Chemistry of Solids 73(2): 302-307.
- Yu J, Yu X (2008) Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ Sci Technol 42(13): 4902-4907.
- Sun JH, Dong SY, Wang YK, Sun SP (2009) Preparation and photocatalytic property of a novel dumbbell-shaped ZnO microcrystal photocatalyst. J Hazard Mater 172(2-3): 1520-1526.
- Zhou M, Yu J, Cheng B (2008) Preparation, characterization and visible- light-driven photocatalytic activity of Fe-doped Titania nanorods and first-principles study for electronic structures. J Hazard Mater B 137: 1838.
- Singhal A, Achary S, Tyagi A, Manna A, Yusuf S (2008) Colloidal Fe-doped ZnO nano crystals: facile low temperature synthesis, characterization and properties. Mater Sci and Eng B 153: 47.
- Prasad TNVKV, Elumalai EK (2011) Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pacific Journal of Tropical Biomedicine 1(6): 439-442.
- Patil RS, Kokate MR, Kolekar SS (2012) Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectro Chimica Acta A 91: 234-238.
- Kumar R, Roopan SM, Prabhakarn A, Khanna VG, Chakroborty S, et al. (2012) Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 90: 173-176.
- Supraja N, Avinash B, Prasad TNVKV (2017) Green synthesis and characterization of silver nanoparticles from Gymnema sylvestre leaf extract: study of antimicrobial activities. Int J Curr Microbiol App Sci 6(3): 530- 540.
- Bonnia NN, Kamaruddin MS, Nawawi MH, Ratim S, Azlina HN, et al. (2016) Green biosynthesis of silver nanoparticles using polygonum hydropiper and study its catalytic degradation of methylene blue. Procedia Chemistry 19: 594-602.
- Vanaja M, Paulkumar K, Baburaja M, Rajeshkumar S, Gnanajobitha G, et al. (2014) Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorganic Chemistry and Applications 742346: 8.
- Supraja N, Prasad TNVKV, Giridhara Krishna T, David E (2016) Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Biochem Biophys Rep 14: 69-77.
- Supraja N, Prasad TNVKV, Giridhar Krishna T, David E (2015) Synthesis, characterization and evaluation of the antimicrobial efficacy of boswellia ovalifoliolata stem bark extract mediated zinc oxide nanoparticles. Appl Nanosci 6(4): 581-590.
- Karunakaran C, Dhanalakshmi R, Gomathisankar P (2012) Phenol-photodegradation on ZrO2. Enhancement by semiconductors. Spectrochim Acta A Mol Biomol Spectrosc 92: 201-206.
- Kansara S, Dhruv D, Joshi Z, Pandya D, Rayaprol S, et al. (2015) Structure and microstructure dependent transport and magnetic properties of sol-gel grown nanostructured La 0.6 Nd 0.1 Sr 0.3 MnO3 manganites: role of oxygen. Appl Surf Sci 356: 1272-1281.
- Sri Sindhura K, Prasad TNVKV, Panner Selvam P, Hussain OM (2013) Synthesis and characterization of phytogenic zinc nanoparticles and their antimicrobial activity. Appl Nanosci 4(7): 819-827.
- Prabha S, Supraja N, Garud M, Prasad TNVKV (2014) Synthesis, characterization and antimicrobial activity of Alstonia scholars bark-extract- mediated silver nanoparticles. J Nano struct Chem 4(4): 161-170.
- Vanaja M, Paulkumar K, Baburaja M, Rajeshkumar S, Gnanajobitha G, et al. (2014) Degradation of methylene blue using biologically synthesized silver nanoparticles Bioinorg Chem Appl 4: 161-170.
- Matinise N, Fuku XG, Kaviyarasu K, Mayedwa N, Maaza M, et al. (2017) ZnO nanoparticles via Moringa oleifera green synthesis: physical properties & mechanism of formation. Appl Surf Sci 406: 339-347.
- Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, et al. (2012) Green synthesis and characterization of silver nanoparticles produced using Arbutus Unedo leaf extract. Mater Lett 76: 18-20.
© 2019 Mosab Nouraldein Mohammed Hamad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






