- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Effect of Plasmodium Falciparum and Plasmodium Vivax on Liver Function Mainly Alanine Aminotransferase and Bilirubin among Known Malaria Patient’s in River Nile State
Mosab Nouraldein Mohammed Hamad*, Huda Badreldin Abdelgadir, Rofida Abd elbage Alhadi, Khadija Eltigani Mohammed, Mozdalifa Babiker Osman and Fatima Awad Salih
Department of Medical Parasitology, Sudan
*Corresponding author: Mosab Nouraldein Mohammed Hamad, Department of Medical Parasitology, Sudan
Submission: September 25, 2018;Published: November 16, 2018

ISSN 2640-9275 Volume2 Issue1
Abstract
The aim of this study was to investigate the effect of malaria on liver functions in known falciparum and vivax malaria patents’ this a hospital-based study, which was carried out at Atbara and Berber towns. A total of 60 samples were included in this study out of which 25 sample of patients with falciparum malaria and 25 sample of patient s’ with vivax malaria and 10 control. Liver function test certainly (ALT) and bilirubin tests were estimated by standard method.
The results showed that an increased level in ALT and bilirubin in malaria patients as compared with control. The highest values of ALT were observed in vivax malaria patient their ages ranged 20-40 years old particularly in male patients. also, younger patients with vivax malaria showed high level of direct bilirubin, in contract the younger and elder falciparum malaria patients showed high level of indirect bilirubin. The elevation of total bilirubin was clearer in falciparum malaria patients’’ particularly in male gender. Although, ALT and bilirubin in normal ranges but in our study the dysfunction of liver is more common in falciparum malaria than vivax malaria.
Keywords: Liver; Malaria; Enzymes; Sudan
Introduction
The term malaria originated from Italian word: meaning bad air and disease was called ague or marsh fever due to its association with swamps in humid regions of the world [1]. It is a term used for acute or chronic infection caused in man or other vertebrates such as mice and donkeys [2]. Malaria is a febrile illness caused by protozoa of the genus plasmodium, transmitted to human by bite of infected female anopheles’ mosquito. Malaria is major public health problem in tropical and temperate areas which is responsible for infecting 300-500 million people and 1-3 million deaths annually [3]. In Africa a child dies from malaria every30 seconds [4].
In spite of phenomenal progress in medical science in latter half of the 20th century, malaria still continues to be a major killer of mankind especially in developing countries [5]. Malaria can be transmitted by three known ways; vector transmission, blood transmission and congenital transmission. Also, the malaria parasite interferes with three major organs in the body, namely: the brain, kidney and liver [6]. The life cycle of all species of human malaria parasite is essentially the same. It comprises an exogenous sexual phase (sporogony) with multiplication in certain female Anopheles mosquitoes and an endogenous asexual phase (schizogony) with multiplication in the vertebrate host. Malaria in Sudan is one of the leading causes of maternal mortality [7].
Liver is a vital organ in humans which can function normally in presence of only 25% of liver parenchyma. Therefore, liver dysfunction is usually masked unless clinical manifestation appears Choudhury et al,2013. They also, stated that Liver function tests are sensitive methods for diagnosing hepatic insufficiency in symptomatic as well as asymptomatic patients. As per the World Health Organization (WHO), malaria due to plasmodium falciparum commonly present with jaundice bilirubin ≥3mg/ dl and involves the liver [8]. Several authors have reported close relationship between incidence of severe malaria and liver damage characterized by jaundice [9,10].
Marsh et al. [11] reported histopathological changes in malaria patients which include hepatocyte necrosis cholestasis, bile stasis and granulomatous lesions. Liver enzymes AST, ALT increase in malaria parasitaemia to level dependent on the degree of parasitaemia and suggest that a liver is involved in the pathophysiology of malaria [12].
Life cycle of malaria parasite (plasmodium)
The life cycle of all species of human malaria parasite is essentially the same. It comprises an exogenous sexual phase (sporogony) with multiplication in certain female Anopheles mosquitoes and an endogenous asexual phase (schizogony) with multiplication in the vertebrate host.
Life cycle in the mosquito
When a female Anopheles mosquito ingests the blood of a person or animal with malaria parasite in the circulation, the asexual forms of the parasite are digested together with the red blood cells while the mature sexual cells (gametocytes) undergo further development [13]. In the stomach of the mosquito, the gametocytes develop into macrogamete and microgamete which are female and male gametes respectively after ex-flagellation. The macrogamete is fertilized by one microgamete to form a zygote (an ookinate). This penetrates the cell wall of the mosquito and lies between the epithelium and the basement membrane [14]. The oocyst nucleus divides several times with the first division being a reduction division to produce several sporoblasts. These divide further to produce thousands of haploid thread-like sporozoites. When mature, the oocyst bursts releasing the sporozoites into the haemocoel of mosquitoes. Some of these sporozoites penetrate salivary gland of the mosquito and are injected with saliva into blood when the mosquito bites man to suck blood (Figure 1 & 2).
Figure 1:Mean of liver enzyme (ALT) in patients malaria infection and control.

Life cycle in the human liver
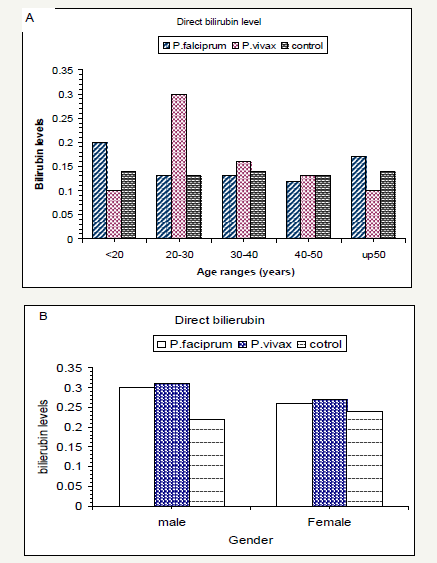
Figure 2:Serum direct bilirubin levels according to subject`s age range(A) and gender (B).
The later phase (Figure 1 & 2) includes the development cycle in the red corpuscles in the blood (erythrocytic schizogony) and the phrasemaking place in the parenchyma cells in the liver (exoerythrocytic schizogony) as reported by [15].
Bilirubin
Bilirubin is formed from the breakdown of erythrocytes and other haem-containing proteins such as myoglobin and cytochromes. The haem (iron porphyrin) of the haemoglobin molecule is separated from the globin and the haem is converted mainly in the spleen to biliverdin which is reduced to bilirubin. This bilirubin is referred to as unconjugated (indirect) bilirubin. It is not soluble in water and cannot be excreted in the urine. It is bound to albumin and transported in the blood to the liver.
In the liver cells, the enzyme glucuronosyltransferase joins (conjugates) glucuronic acid to bilirubin forming bilirubin glucuronides (mainly diglucuronides). This bilirubin is referred to as conjugated (direct) bilirubin It is water-soluble Conjugated bilirubin passes into the bile canaliculi, through the bile duct and into the intestine. In the terminal ileum and colon, the bilirubin is deconjugated and reduced by bacteria to various pigments and colourless chromogens (urobilinogen) most of which are excreted in the faeces as stercobilin. Some of the bilirubin and urobilinogen from the intestine is reabsorbed into the portal circulation and reaches the liver where it re-enters the intestine in the bile and is excreted in the faeces. A small amount of reabsorbed urobilinogen is carried in the blood through the liver and transported to the kidneys where it is excreted in the urine. When exposed to air urobilinogen is rapidly oxidized to the brown coloured pigment urobilin (and stercobilin to stercobilin).
Note: In a healthy individual about 5% of the total plasma bilirubin is conjugated and 95% or more, unconjugated. Urine contains a trace of urobilinogen and no bilirubin.
Value of test: The measurement of serum or plasma bilirubin is usually performed to investigate the causes of liver disease and jaundice, and to monitor a patient.
Liver disorders are often associated with jaundice: Visible jaundice occurs when the concentration of bilirubin in the plasma rises to more than twice its normal limit, i.e. about 34mol/l (2mg%).
The whites of the eyes appear yellow and the skin and body fluids also become pigmented. (Monica part 1).
Alanine aminotransferase
Alanine aminotransferase (ALT) is transferase with enzymatic activity, it catalyses the transfer of an amino group from alanine to α-ketoglutarate with the formation of glutamate and pyruvate. The order terminology was serum glutamic -pyruvic transaminase (SGPT, or GPT).
Tissue source: ALTis distributed in many tissues, with comparatively high concentration in the liver. It is considered the more liver-specific enzyme of the transferases.
Diagnostic significance: Clinical application of ALT assays is confined mainly to evaluation of hepatic disorder. Higher elevations are found in hepatocellular disorders than extra-hepatic or intrahepatic obstructive disorders. In acute inflammatory condition of the liver, ALT elevation is frequently higher than those AST.
Assay of enzyme activity: The typical procedure for ALT consists of a coupled enzymatic reaction using lactate dehydrogenase (LD) as the indicator enzyme, which catalyses the reaction of pyruvate to lactate with the simultaneous oxidation of NADH. the change in absorbance at 340nm measured continuously is directly proportional to ALT activity.
Source of error: ALT is stable for 3-4 days at 4c. It is relatively unaffected by haemolysis.
Reference range: ALT,6 -37 U/L (37c) (clinical chemistry Bishop).
Literature Review
General
Malaria is an infection vector -borne disease having very sign can’t morbidity and mortality rates across the globe [3]. It caused by a plasmodium species namely the plasmodium falciparum, p. vivax, provable, p. malaria, rarely Knowles in humans [16]. liver in involvement in malaria is common in patients of sever malaria. hepatocyte is affected by the malarial sporozoites lead to organ [8].
Malaria disease
Malaria is febrile innless caused by protozoa of the genus plasmodium. also, defined as the presence of one or complication in patients showing high parasitaemia of plasmodium’s in their peripheral blood film [17].
Types of plasmodium’s species
Malaria is febrile illness caused by protozoa of the genus plasmodium five species of plasmodium are responsible for causing malaria in human’s these are plasmodium vivax, plasmodium falciparum, plasmodium oval, plasmodium malaria and plasmodium Knowles [16]. Plasmodium falciparum infection in India is a major cause of sever and complicated malaria, but with the implementation of molecular diagnosis, it has become evident that plasmodium vivax mono infection could also result in multiple organ dysfunction and sever life threatening disease, like plasmodium falciparum infection [18].
Transmission of malaria parasites
Donald & Krogstad [6] reported that the malarial sporozoites once injected in blood by the bite of female Anopheles mosquitoes are attached to hepatocyte through receptor for thrombospondin and properdin. Here these sporozoites become mature to form tissue schizont or become dormant hypnozoites. Tissue schizonts amplify the infection by producing large number of merozoite (10,000 to 30,000). Each merozoite released from the liver can invade a human red blood cell and establishing the asexual cycle of replication in that red cell with the release of 24 to 32 merozoites at the conclusion of 48 to 72 hours asexual cycle. malaria cause abnormalities in the liver however, opinions differ about the clinical importance of this damage [19].
Liver enzymes
Liver dysfunction is a common complication that usually occurs in malaria infection. Some studies have reported a sudden increase in liver enzymes in malaria infected individuals as an indication of liver dysfunctional reported by [6].
Malaria pathogenesis is based mainly on extensive change in biochemical and haematological parameters. Liver function tests are sensitive methods for diagnosing hepatic insufficiency in symptomatic as well as asymptomatic patients Choudhury et al. 2013. Alanine aminotransferase (ALT) catalyses reactions in which the building blocks of protein (amino acid) are transferred from a donor molecule to a recipient molecule .it is found largely in the liver. Hence, it severs as a marker of liver damage which aspartate amino transferase is found in adversity of tissues including liver, muscle, heart, kidney and brain. it is increased of when any of these tissues is injured [20].
SGPT(ALT) was not elevated in plasmodium vivax positive patient s’ showing difference in mean values statistically a highly significant (P< 0.001) in patients having illness of more than two weeks [21]. Also, Suresh & Kotresh [16] stated that serum bilirubin level was raised in 66% of cases showing level s’ above 3.09g/dl in patients with falciparum malaria.
Although, hyperbilirubinemia has been linked with increased malaria related mortality, it is often association with other complication such as acute renal failure or cerebral malaria [9]. Furthermore, Choudhury et al. 2013 showed that, bilirubin was (1.1) value in malarial infection patient s’ relative to (0.7) value in control group. Kauskar et al. reported that, positive correlation of liver enzymes and bilirubin shows that liver function test should be performed along with early diagnosis of plasmodium falciparum malarial infections in order to prevent complication and to reduce mortality. Elabadoiwi et al. reported higher level of ALT, total bilirubin and indirect bilirubin in malaria patients’ group [22,23].
A significant positive correlation coeffect was found between liver enzymes, age and bilirubin level was significantly elevated in the acute falciparum malaria patient s’ than in the non-parasitaemia (SD)of total bilirubin in patients affected with p. vivax was 8.34±3.03 which in p. falciparum was 8.34±4.03 as reported by [24].
Justification
No published data and scanty of information about the effect of predominant plasmodium species on liver enzymes and bilirubin.
Objectives
General objectives: To know the effect of falciparum and vivax malaria on the liver Physiology in local Community, Atbara and Berber Cities, River Nile State.
Specific objectives
A. To measure the level of bilirubin and alanine aminotransferase in known falciparum and vivax malaria pateints.
B. To evaluate the effect of malaria on liver functions and correlation between liver enzyme (alanine aminotransferase ALT and bilirubin) and malaria patients.
Materials and methods
Study design
Case control study
Study area
The study area was community based cross sectional survey. the study area were villages’ and town in the Atbara and Berber towns, River Nile State, Northern Sudan. Patients with falciparum malaria were 25, patients with vivax malaria were 25 and 10 none infected malaria (control) were tested in this study. The clinical data of all cases were gathered as perform an appended. In all cases, detailed history has taken included: Patient s’, name, age, sex. were recorded in requisition form.
Study period
December 2017 - May 2018.
Selection criteria
Inclusion criteria: Falciparum and Vivax malaria patients Exclusion criteria: Patients who having liver cirrhosis, hepatitis, typhoid, diabetic and hypertensive were excluded in this study.
Ethical consideration
all participants informed about the objectives of the proposed research.
Data collection
personal data obtain by using standard questionnaire.
Collection of samples
About 4ml of venous blood was drawn from an antecubital vein through disposable syringe. About 4ml blood was collected in plain vacuum container tube (4ml) from each patient using sterile precaution.
Biochemical tests
Patients’ blood and control blood were collected in plain tube and keep it for (1-2) minutes for clotting once the blood samples become clot centrifuged the blood samples uses laboratory centrifuge serum was separated and proceed for the tests. liver function tests were done.
Following stand recommendation for of the IFCC but was optimized for performance and stability be [25] liver function tests was done using A15 by trained technician under supervision of senior biochemist.
Principle of bilirubin
Direct bilirubin in the sample reacts with 3,5-dichlorophenyl diazonium salt forming a coloured complex that can be measured by spectrophotometry at 535nm3. Both direct and indirect bilirubin couple with diazo in the presence of cetrimide 4,5. The terms direct and total refer to the reaction characteristics of serum bilirubin in the absence or presence of solubilizing (accelerating) reagent. The direct and indirect bilirubin are only equivalent to the conjugate and unconjugated fractions.
Test parameter:
R1: use reagent A
Table 1:
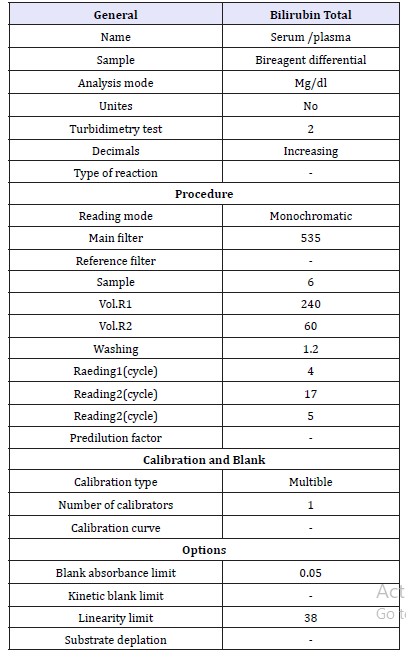
R2: use reagent B (Table 1)
Principle of ALT: Alanine aminotransferase (ALT or GPT) catalyzes the transfer of the amino group from alanine to 2-oxoglutarate, forming pyruvate and glutamate. The catalytic concentration is determined from the rate of decrease of NADH, measured at 340 nm, by means of the lactate dehydrogenase (LDH) coupled reaction 1,2,3,4.
Alanine + 2 - Oxoglutarate →Pyruvate + Glutamate
Pyruvate + NADH + H+→ Lactate + NAD+
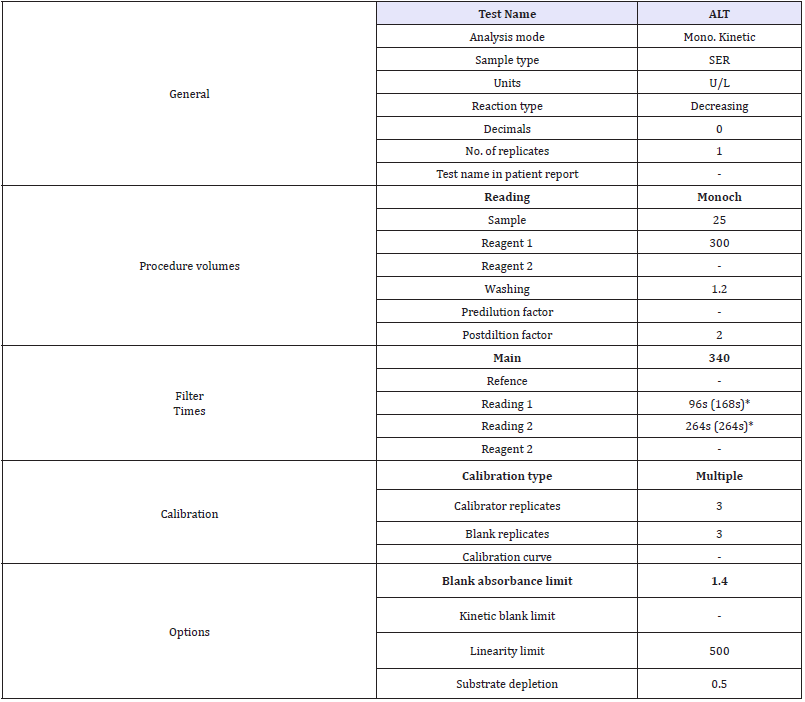
Assay Parameters (Table 2)
Statistical analysis
Table 2:
The data obtained were analysed using statistical analysis computer software (SPSS) according to analysis of variance (ANOVA) and individual comparisons of the group mean values were done using least significant difference (LSD) with 5% level of probability. Also, correlation coefficient between parameters was observed using SPSS version 21 taking p value significant when less than 0.05.
Result
Elevation of ALT liver enzymes in malaria and nonmalaria volunteers
Analysis of variance showed significant effects (p< 0.01) on liver enzyme ALT in tested groups (Table 3). There was significant increase in mean of ALT in malaria patients as compared with control group (Table 4). Although the values of this parameter were in normal ranges, but younger and elder falciparum malarial infection patients and control group showed high ALT values. while the highest values of ALT were observed in vivax malaria patient s their ages ranged between 20-40 years old (Figure 1A& 3).
Table 3:Mean squares of ALT, serum Bilirubin in malaria infection and control subjects.
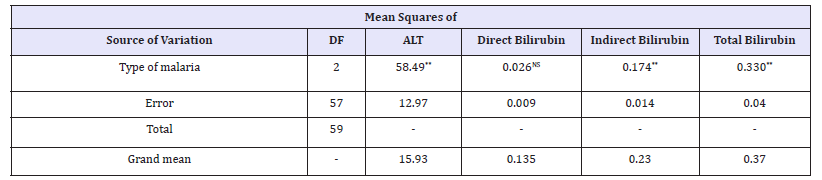
**: significant at 1% level of probability, NS: none- significant of probability.
Table 4:Means of ALT, serum Bilirubin in malaria infection and control subjects.
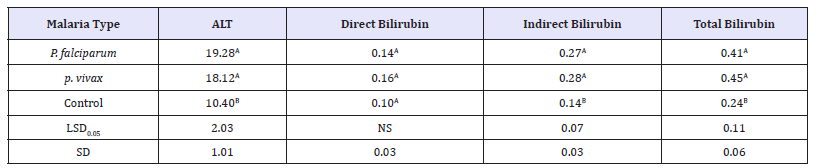
LSD: Least Significant Difference; SD: Standard Deviation
Means followed by the same letter in same column were none-significant different from each other according to Least Significant Difference at 5% level of probability.
Means followed by the same letter in same column were none-significant different from each other according to Least Significant Difference at 5% level of probability.
ALT values according to gender, falciparum malaria patients and control group showed high values, but the trend was inverse in vivax malaria infection where male patients showed higher ALT values than female patients (Figure 1B & 3).
Elevation of serum bilirubin analysis in malaria and non-malaria volunteers
Statistical analysis showed highly significant effect a serum indirect and total bilirubin between malaria infection patients and control group (Table 3). Although the values of these parameters were in normal ranges. The indirect and total bilirubin values (0.29 and 0.45) in vivax malaria and (0.27 and 0.41) in falciparum malaria patients were higher than these lower values observed in control group (Table 4).
Although direct bilirubin was not significant, but the levels were observed in vivax malaria patient’s their age between 20-30 years old particularly in male patient’s (Figure 2A, 2B & 3). Regarding indirect bilirubin the younger falciparum malaria patients showed the highest level followed by elder patient’s, but the trend was inverse for vivax malaria patient’s where the higher values were observed in patient s’ their age ranged 30-40 years particularly in female patients (Figure 3A,3B).
The elevation of younger and elder was clearer in falciparum malaria patients particularly in male gender. which the level of total bilirubin was recorded in vivax malaria infection patient s’ their age between 20-40 years old particularly in male gender (Figure 3 & 4).
Figure 3:Serum indirect bilirubin levels according to subject’s age range(A) and gender (B).
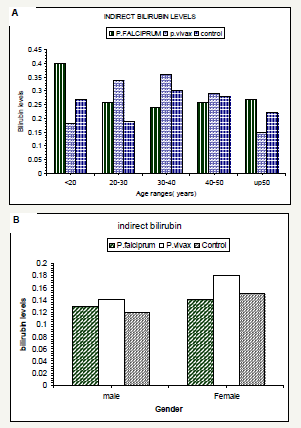
Figure 4:Life cycle of plasmodium.

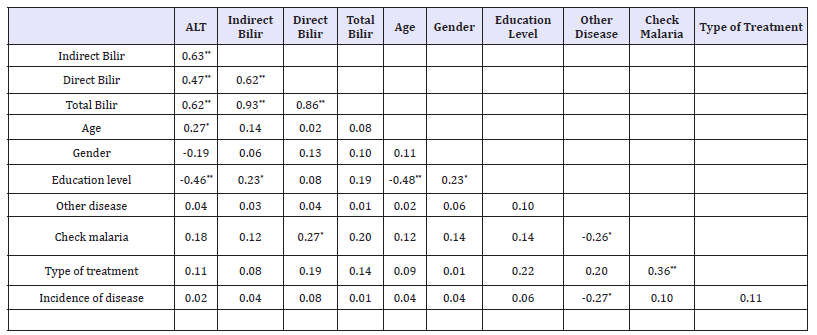
Correlation analysis
Data presented in Table 5 indicated significant and positive correlation between ALT, all bilirubin, age of malaria patient’s infection and control group. but there was negative significant correlation between education level with both ALT and age but positive significant correlation with gender of volunteer in this study also, there were significant negative correlation between other disease with both check malaria and incidence of disease. However, types of treatments were significantly correlated with check malaria only [26-30].
Table 5:Correlation coefficient between means Liver function enzyme (ALT), Serum bilirubin.
Discussion
ALT parameter measured in this study in normal ranges, However, in the present study, it was observed that the liver enzyme (ALT) level were elevated in both falciparum and vivax malaria patients when compared with control group. The observed increase in ALT could be due to leakage form hepatic cells that were injured by the auto-immune progress and or by abnormal cell activation induced by the parasite. The finding of the present study correlated with finding of previous studies of Guthrow et al. [11]. In vivax malaria and Sudha et al. [31]. In this regard, as liver is vital organ in humans, it receives highest amount of blood supply which account for 25% of the total cardiac output. encounter of any foreign substances entering our body with hepatocytes is earlier than any other cells. Liver dysfunction is common in hepatic and non-hepatic injuries. Since liver is involved in the life cycle of malaria parasites. Oluwole et al. [24] reported that most of patients’ showed elevation in (ALT) enzyme indicating liver damage.
These results were agreement with results obtained in this study where both falciparum and p. vivax infection patients’ where showed elevation in ALT enzyme as compared to control group. In addition, ELbadawi et al. [9] in Sudan reported the higher levels of ALT in malaria infection patients. The observed elevation of serum bilirubin levels recorded in this study in malaria patient s’ indicated increased of red blood cell haemolysis these finding were confirmed by these results reported by many authors Renner [27].
Stated that elevated serum bilirubin has been associated with hepatocellular damage, biliary tract obstruction, haemolysis and neonatal jaundice while Oluwole et al. [24] reported that, elevation of serum bilirubin level in malaria patients indicated increased red blood cell all haemolysis. Also, ELbadwai et al. [9], Singh et al. [30] reported supporting evidence. the higher level of bilirubin in males’ patient indicated the greater risk for male s’ which might be due to the greater exposure to the day biting vector mosquito [29- 31] at their work places. This was further confirmed by significant correlation between liver enzyme (ALT), bilirubin and age of patient s’ observed in this study. The positive significant correlations between these characters indicated their unlimited contribution to liver function diagnosis [32,33].
Conclusion
Our study concluded that, although ALT and bilirubin levels within normal range, malaria have significant impact on liver function test. Also, the dysfunction of liver is more common in falciparum malaria than vivax malaria.
Recommendation
Based on the above findings our recommendations are:
1. Further studies must be conduct with large sample size.
2. Liver function tests (LFTs) should be performed along with early diagnosis of both falciparum, p. vivax infection s’ in order to prevent complicated and reduce mortality.
3. Further studies must be carried out, regarding plasmodium density.
4. Laboratory technologist must identify plasmodium species in order to cure the patient and prevent further complications which may occur due to dormant stages in vivax malaria and subclinical stages in falciparum malaria.
References
- Adam I, Tarning J, Lindegardh N, Mahgoub H, McGready, et al. (2012) Pharmacokinetics of piperaquine in pregnant women in Sudan with uncomplicated plasmodium falciparum malaria. Am J Trop Med Hyg 87(1): 35-40.
- Adeosun, OG, Oduola T, Akanji (2007) Biochemical alternation in Nigerian children with acute falciparum malaria. Afr J Biotechnol 6(7): 881-885.
- Michael LMS, David Tory (1985) Clinical chemistry bishop. Enzyme clinical significance, China, pp. 270-278.
- Casles PC, Reports D (2004) Malaria and the red cell. Vox sanguinis 87(2): 115-119.
- Cheesbrough M (1992) Direct laboratory practice in tropical countries. part 2. (2th edn), Cambridge Univ-press, UK, p. 454.
- Donald H, Krogstad J (1995) Plasmodium species (Malaria). Mandell Douglas and Bennett’s. (5th edn), pp: 2817-2830.
- Dondrop AM, Day NP (2007) The treatment of sever malaria. Tans Soc. Trop Med Hug 101: 633-634.
- ECCLs (1989) Determination of the catalytic activity concentration in serum of L-aspartate amino transferase Klinische chemise Mi ltelungen 20: 198-204.
- ELbadawi NEE, Mohamed MJ, ELZaki N, Mohamed AA, Ounsa GE, et al. (2012) The effect of malaria on biochemical liver function parameters in sudanese pregnant women. J Physiobiochem Metab 1: 2.
- Francis N, Warrel DA (1993) Pathology and pathophysiology of human malaria: In: Chwatt’s B (Ed.), Essential Malariology. Edward Anold Kondon, London, UK, pp. 49-59.
- Guthrow CE, Morris JF, Day JW (2007) Enhanced none-enzymatic of human serum albumin. Proc Natl Acad Sci U S A 76(9): 4258-4261.
- Hasan, Fam, Owyed S (2003) Interpretation of liver of chemistry tests; Bulletin of Kuwait Institute for Medical Specialization 2: 27-310.
- Human malaria. In: Chwatt’s B (Ed.), Essential malariology. Edward Anold Kondon, UK, pp. 49-59.
- Joy D, Feng X, Mu J (2003) Early origen and recent expansion of plasmodium falciparum Science 56(17): 300-321.
- Kausar MW, Moeed kh, Asif N, Rizwi F, Raza s (2010) Correlation of bilirubin with liver enzymes in patients of falciparum Malaria. Int J Pathology 8(2): 63-67.
- Kotresh N, Suresh N (2016) Liver function abnormalities in falciparum malaria. In J of Advance in Medicine 3(4): 847-850.
- Long M (1999) The family encyclopedia of medicine and health Robinson, London, UK, p. 493.
- Maduka C, Emeka EN, Blessing NE (2008) Effect of malaria parasitemia on liver enzymes test. International Journal of Tropical Medicine 3: 49- 52.
- Marsh K, Foster D, Waxuiru C, Mwangi I, Newton C, et al. (1995) Indication of Life the threatening malaria in Africa Children. N Eengl J Med 332(21): 1399-1404.
- Mishra SK, Mohantys S, Das BS (1992) Hepatic Change in Plasmodium falciparum. Indian J Malaria 29(3): 167-171.
- Monica CM (2005) District laboratory practice in tropical countries clinical chemistry tests. America Cambridge University, New York, USA, pp. 349-355.
- Mueller I, Zimmerman PA, Reeder JC (2007) plasmodium malaria and plasmodium oval-The bashful malaria parasites, trends parasital 23(6): 278-283.
- Nnodim JK, Nwannjo HU, Opra AU (2010) Blood glucose level and liver enzymes activities in malarial patients in Owerri. J of Medical Lab Sci1(1): 7-9.
- Oluwole O, Sia S, Mohmmed M (2010) Plasmodium falciparum-induced kidney and liver dysfunction in malaria patient sin free town, sherlene. J of Biochemical Research 2(1): 70-74.
- Onyesmo I, Onye Makonor N (2011) Levels of parasitemia and changes in some liver enzymes among malarial infected patients in edo-delta region of Nigeria. Current Research J of Biological sciences 3(2): 78-81.
- Philips RS (2001) Current status of malaria and potential for control. Clinical Microbiological Reviews 14(1): 208-226.
- Renner EL (1995) Liver function tests. Bailliere J Clin Gastrin enterology Med 40: 130-134.
- Shukla A, Kulkarni CV, Rangnekaw A (2018) Liver of parasitemia and its relation to hematological parameters and liver function among patients with falciparum malaria, a study from central India periapex. Indian J of Research 7(7): 67-68.
- Sina B (2008) Focus on Plasmodium vivax. trend parasite 18: 287-309.
- Singh G, Urhokar AD, Maheshwari U, Sament P (2018) Role of liver enzymes in patient’s infected with Plasmodium vivax and plasmodium. Falciprum Int J Microbiology Health Res 2(1): 50-54.
- Sudha J, Shrestha S, She G, Gole G (2014) Assessment of serum bilirbin and hepatic enzymes in malaria patients. Int J of Biomedical and Adance Res 5(3): 3013-3016.
- Yokoto USC, Calisei T (2006) Malaria parasite and their relationships with host. Malaria Res 44: 265-373.
- Yuda M, Ishino T (2004) Liver invasion by malarial parasites-how do malarial parasites break through the host barrier? Cellular Microbiol 6(12): 1119-1125.
© 2018 Mosab Nouraldein Mohammed Hamad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






