- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Frequency of Anemia and Possible Risk Factors Among Sudanese Children with End Stage Renal Disease
Abdelmageed AS*, Abdelrahim M, Muddathir and Rashid A Ellidir
Soba University Hospital, Sudan
*Corresponding author: AbdElmageed A Sobahi, Soba University Hospital, Sudan
Submission: September 25, 2018;Published: November 12, 2018

ISSN 2640-9275 Volume2 Issue1
Abstract
Background: Anemia is a common Feature of chronic kidney disease, but the management of anemia in children is complex. Erythropoietin and Supplemental iron are used to maintain hemoglobin levels. The aim of this study to determine the Frequency of anemia and possible Risk Factors Among children with End stage renal disease.
Methods: A total of 96 children, 61males (63.5%) and 35 Females (36.5%), were attended at hemodialysis units in Khartoum state were enrolled in the study and Frequency of anemia was estimated by analyzing CBC on blood counter (sysmex). The concentration of iron profile, C-reactive protein and parathyroid hormone was measured using COBAS INTEGRA 400 PLUS and COBAS E411.
Results: 99% of children were anemic, 4.17% of them were suffering from iron deficiency anemia and there are other causes contributing to anemia in ESRD patients which are inflammation and hyperparathyroidism.
Conclusion: The prevalence of anemia in children on hemodialysis in Sudan appears to be higher than that reported in other studies despite extensive use of rHuEPO and iron supplementation.
Keywords: Hemodialysis; Anemia; Erythropoietin; Hemoglobin; Iron; Parathyroid hormone; Children; Khartoum; Sudan
Introduction
Anemia is major complication of end –stage renal disease (ESRD) in children [1]. When severe, it is associated with cardiovascular dysfunction, cardiomyopathy, and death [2] Correction of anemia in children with ESRD improves cardiac dysfunction exercise tolerance and reduces left ventricular hypertrophy [2]. approximately 25% of adult patients maintained on chronic hemodialysis have anemia. Anemia is defined by the National kidney Foundation Dialysis Outcome Quality initiative (K/ DOQI) as a hemoglobin value less than 11g/dl [3]. More than 75% of children with anemia maintained on chronic hemodialysis exhibit signs of left ventricular hypertrophy, a harbinger of cardiovascular morbidity in adulthood [4]. The major cause of anemia in patients with chronic kidney disease and end stage renal disease (ESRD) is erythropoietin (EPO) deficiency, resulting from its decreased production from the kidneys [2]. The remarkable development and subsequent introduction of recombinant human erythropoietin (rHuEPO) in 1989 made it possible to safely and effectively treat the anemia of renal insufficiency and practically eliminate the need for repeated transfusion [5] Despite the advances in dialysis care and the use of erythropoietin, anemia continues to be a clinical problem seen in patients with ESRD [3]. It was believed that iron deficiency was the major predictor of EPO hypo responsiveness [6]. Despite the extensive use of erythropoietin and iron supplements, over one third of children aged between 12 to< 18 maintained on chronic hemodialysis have a mean hemoglobin of less than 11g/dl [6]. Other factors that have been shown to influence the response to rHuEPO in adult and pediatric patients on dialysis include dosage, route of administration, acute of chronic infection and aluminum intoxication [1]. Refractory anemia appears to be more common in those patients on dialysis who also suffer from protein-energy malnutrition (PEM) or inflammation [6]. Secondary hyperparathyroidism contributes to resistance of rHuEPO in adults [1]. Craig et al showed when serum PTH were markedly elevated in pediatric patients, response to rHuPO will be poor [1].
Rationale
Accurate diagnosis of anemia and iron deficiency is essential in hemodialysis patients, since these conditions are prevalent during chronic disease. Understanding the etiology of anemia and iron deficiency in hemodialysis patients can help health care providers in managing the anemia, which in turn improves their quality of life. Also, there is no published data regarding this study in Sudan.
Objectives
General objective
To determine Frequency of Anemia, iron deficiency anemia and Possible Risk Factors among Children with End-stage of Renal Disease attending Pediatric Hemodialysis Units in Khartoum State.
Specific objectives
A. To determine frequency of anemia by measuring hemoglobin concentration for children with end-stage of renal disease.
B. To determine the presence of iron deficiency anemia among anemic children by measuring iron profile.
C. To find out if there is any correlation between (duration of hemodialysis, level of PTH and level of CRP) and degree of anemia.
Material & Methods
A cross sectional study was conducted in at Soba University hospital, Khartoum children hospital and Omdurman children hospital. Khartoum, Sudan during the period of January 2014 to August 2016.The study population consisted of 96 patients on chronic hemodialysis from pediatric hemodialysis centers. All the patient-specific parameters were recoded, including Age, gender, duration of dialysis (6 month vs.6 months or longer). Blood studies were performed immediately pre-dialysis. Complete blood count, serum iron, ferritin, total iron binding capacity, Transferrin, Transferrin/saturation %, Intact parathyroid hormone (PTH), C-reactive protein were measured. Data on patient rHuEPO dose (unit/Kg/week), and oral iron (mg/Kg) or administration of intravenous iron (mg/Kg/week) were obtained from dialysis charts. All routine laboratories measurements were performed by Sysmex Kx- 21 N using automated methods. Serum Iron profile, intact PTH and CRP were performed by COBAS INTEGRA 400 Plus and COBAS E411. Serum CRP was obtained to indicate the presence of an inflammation state. Anemia was defined as a hemoglobin value less than 11g/dl and severe anemia defined as hemoglobin value less than 8g/dl. Iron deficiency was defined as ferritin ≤100ng/dl or the percentage transferring saturation less than 20% and mean corpuscular (MCV) less 75fl. Serum intact PTH>200pg/ ml was considered as high turnover bone disease secondary to hyperthyroidism. The results were analyzed using the statistical Package for Social Sciences (SPSS) program and expressed as mean and standard deviation.
Ethical clearance
The study received ethical clearance from the Research Board at Faculty of Medical Laboratory Sciences, University of AlZaiem AlAzhari, after that the approval from health authorities at the ministry of health in Khartoum was obtained.
Results
The Study group was composed of patients Aged between (5-17 years) (31 Patients were less than 12 years and more 65 patients more than 12 years). The mean age of patients on hemodialysis was 13.2±3.2 years. There were 61 males (63.5%) and 35 females (36.5%). Mean duration of hemodialysis was 28.7±25.6 months (Table 1). Packed cell volumes of most of the patients were less than normal. The mean hemoglobin was 7.38±1.86 (Table 1). Thirty-two patients (34%) had hemoglobin value less than 11g/dl (anemia) and 65% (62 patients) had hemoglobin value less than 8g/dl (severe anemia) (Table 2). One of 4.17% of anemic children had mean transferrin saturation (TSAT) less than 20%. Absolute iron deficiency (defined as a TSAT of less than 20% and a ferritin less than 100ng/ml) was only seen in 4 patients (Figure 1 & Table 3).
Figure 1:Frequency of anaemia and IDA among children with End stage of renal disease.

Table 1:Mean of age, duration of hemodialysis and hematological parameters among children with End stage of renal disease.
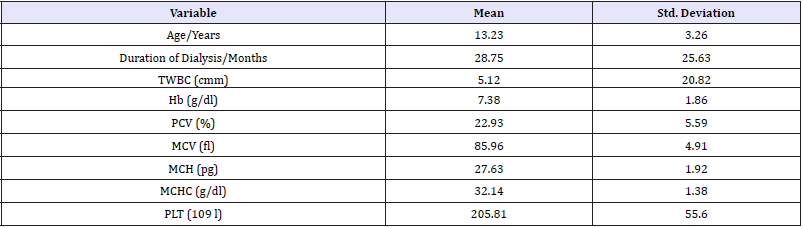
Table 2:Frequency of anemia and IAD according to the severity of anemia.
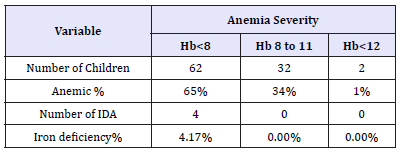
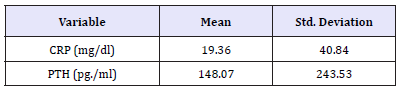
Table 3:Mean of C-reactive protein and PTH among children with End stage of renal disease.
Discussion
Despite the extensive use of erythropoietin and prescribed iron supplements, over 99% of children maintained on chronic hemodialysis and a mean hemoglobin less than 11g/dl. Patients new to dialysis (treated less than 6 months) were more anemic. Frankenfield et al. [3] showed that 37% (160/435) of HD patients aged between 12 and less than 18 years were anemic in the National 2001 ESRD Clinical Performance Measures Project. Fadrowski et al. [3] demonstrated that more than one third of pediatric patients on dialysis were anemic during the project years of 2000 and 2001 [7]. The 2001 North American Pediatric Renal Transplant Cooperative Study annual report showed that 63% of 1855 pediatric patients on chronic dialysis who were receiving rHuEPO at 6 months of dialysis had hematocrit values of ≤33%(≤11gldl) [8]. In our study more than 99% of patients were anemic. The recommended starting dose of rHuEPO is 50-150U/Kg given three times weekly [2]. Our patients received a mean weekly rHuEPO doses of 158U/Kg, these doses were significantly higher than those received by adults.
Fadrowski et al. [3] showed that an increase in age and dialysis for less than 6 months were 2 factors that enhanced anemia [8]. There was no correlation between age, gender and anemia in our study but patients new to dialysis (treated less than 6 months) were more anemic. Some patients did not respond to rHuEPO therapy even if high doses were used. The main reason for EPO resistance is iron depletion or insufficient access to iron depletion or insufficient access to iron storage pools [9]. Almost all our patients had iron supplements. There are no standards for iron adequacy in concentration >100ng/ml and percentage transferrin saturation of >20% [10]. Serum ferritin >10ng/ml and percentage transferrin saturation as low as 7% are considered normal for healthy children [11]. Serum ferritin >40ng/ml has been reported to be adequate in children on dialysis [12]. According to protocol of adult on dialysis only 4 of our patients had iron deficiency anemia. Severe secondary hyperparathyroidism appears to be an important factor in the severity of anemia in children with chronic renal failure [1]. PTH may be a direct inhibitor of endogenous erythropoietin production [13].
Another mode of action of PTH in ESRD is an increase in red blood cell osmotic fragility, leading to a decrease in red blood cell survival time [14]. Synthetic PTH or serum from hyperparathyroidism patients has been reported to inhibit red blood cell precursors in vitro in some studies [15]. Likewise, hyperparathyroidism may also affect anemia by causing bone marrow fibrosis which reduces the available space for erythroid- forming units [16]. A serum PTH level at 200pg/ml has been shown previously to be strongly predictive of osteitis fibrosa in children [17]. PTH effect on erythropoiesis can be overcome by higher doses of rHuEPO. PTH level at more than 200pg/ml was seen in 14 our patients and 10 of them had PTH level at more 400pg/ml in our study. Several previous studies reported an association between anemia and inflammation in patients on dialysis. Reflected by a high serum concentration of CRP [18]. More cover, IL and TNF-alpha have been shown to inhibit EPO production in vitro and have a suppressive effect on erythropoiesis [19,20]. Uptake of iron is lower than normal in inflammation [21]. Serum level of ferritin a marker of iron stores and appositive acute-phase reactant have been shown to be paradoxically high in patients with ESRD with refractory anemia [22,23]. Increased ferritin production may prevent iron delivery to erythrocyte precursors [22]. Finally, patients with inflammation may be more prone to gastrointestinal bleeding [21]. In this study inflammation existed in 15 patients in which serum ferritin was more than 2000ng/ml in 8 of them.
Conclusion
Frequency of Anemia is high despite of EPO therapy. Frequency of iron deficiency anemia is less compared to Anemia as most of the patients were in iron therapy. Sever hyperthyroidism, malnutrition and inflammation should be considered as other causes (risk factors) of anemia in this study. The results of this study indicate the need for continued improvement in the management of anemia in children undergoing chronic hemodialysis.
Acknowledgement
We are very thankful to all patients participated, to their families, to Pathology department staff at SUH and to Mr. Mosab Nouraldein.
References
- Belsha CW, Berry PL (1998) Effect of hyperparathyroidism on response to erythropoietin in children on dialysis. Pediatr Nerphrol 12(4): 298- 303.
- Chavers BM, Roberts TL, Herzog CA, Collins AJ, St Peter WL (2004) Prevalence of anemia in erythropoietin-treated Pediatric as compared to adult chronic dialysis patients. Kidney Int 65(1): 266-273.
- Frankenfieid DL, Neu AM, Warady BA, Barbara AF, Curtis AJ, et al. (2003) Anemia in pediatric hemodialysis patients: results from 2001 esrd clinical performance measures project. Kidney Int 64(3): 1120-1124.
- Mitsnefes MM, Daniels SR, Schwartz SM, Meyer RA, Khoury P, et al. (2000) Sever left ventricular hypertrophy in pediatric dialysis, prevalence and predictors. Pediatr Nephrol 14(10-11): 898-902.
- Warady BA, Marin HO (2003) Morbidity and mortality in children with anemia at initiation of dialysis. Ped Nephrol 18(10): 1055-1062.
- Kalantar ZK, Allister CJ, Lehn RS, Nissenson AR, Kopple JD, et al. (2003) Effect of malnutrition inflammation complex syndrome on EPO hypo responsiveness in maintenance hemodialysis Patients. Am J Kidney Dis 42(4): 761-773.
- Fadrorski JJ, Furth SL, Fivush BA (2004) Anemia in pediatric dialysis patients in end-stage renal disease network 5. Pediatr Nephrol 19(9): 1029-1034.
- Neu AM, Ho PL, Donald RA, Warady BA (2002) Chronic dialysis in children and adolescents the 2001 NAPRTCS Annual Report. Pediatric Nephrol 17(8): 656-663.
- Tenbrock K, Berghaus MJ, Michalk D, Querfeld U (1999) Intravenous iron treatment of renal anemia in children on hemodialysis. Pediatr Nephrol 13(7): 580-582.
- Ad Hoc Committee for the National kidney Foundation (1989) Statement on the clinical use of recombinant erythropoietin in anemia of end-stage renal disease. Am J Kidney Dis 14(3): 163-169.
- Koerper MA, Dallman PR (1977) Serum iron concentration and transferrin saturation in the diagnosis of iron deficiency in children: normal developmental changes. J Pediatr 91(6): 870-874.
- Wiefel MDE, Waldherr R, Feist D, Kaick GV (1984) The assessment of iron stores in children on regular dialysis treatment .Contrib Nephrol 38: 141-152.
- Urefia P, Eckardi k, Sarfati E, Zingraff J, Zins B, et al. (1991) Serum erythropoietin and erythropoiesis in primary and secondary hyperparathyroidism,effect of parathyriodectomy. Nephron 59(3): 384- 393.
- Bogin E, Massy SG, Levi J, Djaldeti M, Bristol G, et al. (1982) Effect of parathyroid hormone on osmotic fragility of human erythrocyte. J Clin Invest 69(4): 1017-1025.
- Meytes D, Bogin E, Ma A, Massry SG, Dukes PP (1981) Effect of parathyroid hormone on erythropoiesis. J clin Invest 67(5): 1263-1269.
- Zingraff J, Drüeke T, Marie P, Man NK, Jungers P, et al. (1978) Anemia and secondary hyperparathyroidism. Arch Intern Med 138(11): 1650-1652.
- Salusky IB, Ramirez JA, Oppenheim W, Gales B, Segre GV, et al. (1994) Biochemical markers of renal osteodystrophy in pediatric patients undergoing CAPD/CCPD. Kidney Int 45(1): 253-258.
- Barny P, Divino FJC, Bergstrom J (1997) High creactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 29(4): 565-568.
- Jelkmann W, Pagel H, Wolff M, Fandrey J (1992) Monokines inhibiting erythropoietin production in human hepatoma cultures and in isolated perfused kidneys. 50(4): 301-308.
- Means RT, Krantz SB (1992) Progress in understanding the pathogenesis of the anemia of chronic disease. Blood 80(7): 1639-1647.
- Stenvinkel P (2001) The role of inflammation in the anemia of end-stage renal disease. Nephrol Dial Transplant 7: 36-40.
- Zedeh KK, Don BR, Rodrigues RA, Humphreys MH (2001) Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am J Kidney Dis 37(3): 564-572.
- Zadeh KK, Luft FC, Humphreys MH (1999) Moderately high serum ferritin concentration is not a sign of iron overload in dialysis patients. Kidney Int 56(2): 758-759.
© 2018 Abdelmageed AS. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






