- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Extracellular Synthesis of Calcium Precipitating Bactria Isolated From Rock Environment Region and its Characterization Studies
Supraja N2 and TNVKV Prasad1*
1,2 Nanotechnology Laboratory, Acharya NG Ranga Agricultural University, India
*Corresponding author: TNVKV Prasad, Nanotechnology Laboratory, Regional Agricultural Research Station, Acharya NG Ranga Agricultural University, Tirupathi-517507, AP, India
Submission: May 04, 2018; Published: May 29, 2018

ISSN 2640-9275Volume1 Issue3
Abstract
The Bacillus sp has a calcium carbonate crystal formation competent this isolated from rock environment. This calcium precipitating bacterium was identified by biochemical test. In this study, a microbiological aspect of calcium carbonate crystals the nature of crystal deposits of bacteria has been explained. The biogenic calcium crystals formations in CPB were analyzed by XRD, FT-IR method. It is claimed that the temperature is one of the causative factor for calcium carbonate crystals formation and in rock environment.
Introduction
Calcium carbonate precipitation is a common phenomenon found in environment such as marine water, fresh water and soil. Microbial associations with natural carbonate deposits have been described for seawater [1], saline lakes [2] and soils [3]. The occurrence of human kidney stone development [4], as well as the deposits on the Martian meteorite [5] and the phenomenon of nano forms were hypothesized to be in association with microbial calcification [6]. But no study has been carried out on calcium precipitation by bacteria at high temperatures. In this study, the occurrence of crystal formation in rocks has been reported. The identified isolates showed positive for urease activity. The result supports the observation made by previous investigators [7,8] who noticed urease positive in CPB. Calcium carbonate (CaCO3) precipitation is a common phenomenon found in the environment such as marine water, fresh water and soils. It is a rather straight forward chemical process governed by four key factors: viz. calcium (Ca2+) concentration, the concentration of dissolved inorganic carbon (DIC), pH, and availability of nucleation sites. Boquet et al. [4] suggested that almost all bacteria are capable of CaCO3 precipitation. In this study, it is possible that the identified species may consume calcium acetate and produce organic acids which results in a reduction of pH. The low pH shifted to higher side as per the following reaction. In the presence of bacterial system, there is no significant gradual reduction of the pH. In the present investigation, the rock samples were collected from Tirupathi, (Chandragiri region) Andhra Pradesh, India. The samples were collected from rock environment with the help of sterile spatula taken in the sterile container for identification of calcium carbonate crystals.
Experimental Materials and Methods
Sample collection (cpb)
The rock samples were collected from Tirupathi, (Chandragiri region) Andhra Pradesh, India. The samples were collected with the help of sterile spatula taken in the sterile container. The collected samples were in crystal form and the samples were grained with the help of mortar and pestle to bring those samples to powder form. These samples were stored in an ice box and transported for microbiological characterization to Nanotechnology Laboratory, India for further analysis.
Sample collection
The rock samples were collected from Tirupathi, (Chandragiri region) Andhra Pradesh, India. The samples were collected with the help of sterile spatula taken in the sterile container. The collected samples were in crystal form and the samples were grained with the help of mortar and pestle to bring those samples to powder form. These samples were stored in an ice box and transported for microbiological characterization to Nanotechnology Laboratory, India for further analysis.
Bacteriological Analysis of Scale
Isolation of calcium precipitating bacteria from rock samples
Rock samples (1ml) were taken, suspended in 9ml sterile saline solution in a test tube and vortexed. The samples were subjected to serial dilution and plated on B4 medium (g/l): Calcium acetate 2.5: Yeast extract 4.0 and Glucose 10 [4]. 1ml of sample was taken in a petriplate and B4 medium was added thoroughly by rotating the plate in clockwise and anticlockwise direction and allowed to solidify. Then the inoculated plates were incubated at 37 °C for 2-3weeks. The total viable bacterial counts were enumerated, and the bacterial population was expressed as colony forming units per gram (CFU/gm).
Partial biochemical characterization of the isolates
Morphologically dissimilar dominating isolated colonies were selected randomly, further streaked on B4M agar plates and purified. The pure cultures were maintained in B4M agar slants at 4 ᵒC to keep the microbial strain viable. The dissimilar aerobic bacteria isolated from medium were identified according to Bergeyʼs Manual of Determinative Bacteriology.
The isolated bacterial cultures were identified up to genus level by their morphological and partial biochemical characterization using the following tests shown in Table (1).
Table 1: Biochemical Test for calcium precipitating bacteria isolated from rock environment region.
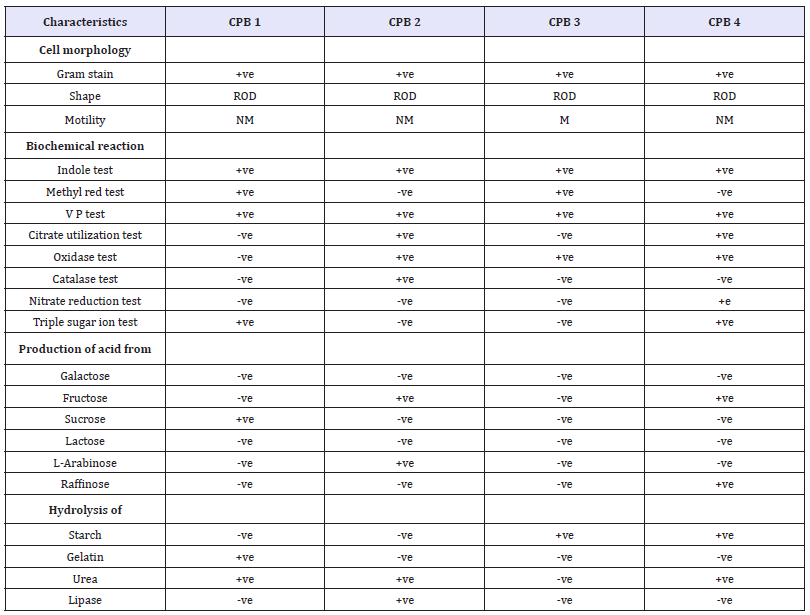
Note: M-Motile, NM- Non motile, AB/AS-Acid Butt, Alkaline Slant, +, - positive, Negative.
a. Gram staining
b. Catalase test
c. Oxidase test
d. Pigment production
e. Nitrate reduction test
f. Indole production
g. Methyl red test
h. Voges- Proskauer test
i. Citrate utilization test
j. Mc Conkey test
k. Urease activity
l. Starch hydrolysis test and
m. Carbohydrate fermentation test
In addition, citrate agar was used to detect the iron-reducing activity of the isolates. Bio-chemical characterization of the isolates was carried out employing Hi-media biochemical test kit (Mumbai) according to the manufacturer’s instructions.
Characterization of Calcium Precipitating Bacteria
X-ray diffraction (XRD) and FTIR analysis:
The CPB was inoculated in to B4 medium and incubated for 2-3weeks at 50 °C and 60 °C. The cultures were centrifuged at 6000rpm for 30min, which form slime layer sediment (pellet) in the bottom (white color) and allowed to air dry. These sediment samples were characterized employing XRD and FTIR. The calcium carbonate crystal samples collected from rock samples and analyzed by XRD and FTIR. The XRD model: Xpert PRO PAN, X-ray diffractometer with Syn Master 793 software to identify the calcium carbonate crystal. The XRD pattern was recorded using computer controlled XRD-system, JEOL, and Model: JPX-8030 with CuK radiation (Ni filtered=13418Ao) at the range of 40kV, 20A. The ‘peak search’ and ‘search match’ program built in software (Syn master 7935) was used to identify the peak table and ultimately for the identification of XRD peak. The FITR spectrum taken in the mid IR region of 400-4000cm-1. The spectrum was recorded using ATR (attenuated total reflectance) technique. The sample was directly placed in the KBr powder and the spectrum was recorded in the transmittance mode.
Result and Discussion
The Bacillus sp (40 °C) calcium precipitating bacteria were isolated from rock region collected from Chandragiri Figure 1. The total viable bacterial count was 5.2×106 CFU/ml. The morphologically different isolates were obtained in the sample. These cultures were used for further study. All these isolates were characterized by biochemical tests Table 1. The selected isolates were grown at 40 °C temperature. In control system at Figure 2, a peak noticed at 3346 and 1460cm-1 was to the C-H stretching vibration of aromatic and alkynes compounds. A peak observed at 2906cm-1 indicates the N-H stretching vibrations. A peak observed at 2115 and 1166cm-1 indicates CaCO3 stretching vibration of metallic carbonates. A peak observed at 1743 and 1637cm-1 indicates carboxylic dimer stretching vibration of metallic carbonates. A peak observed at 1362cm-1, indicated strong amide II bond which may be due the adsorption of bacterial protein and calcium crystals. A peak observed at 1043cm-1 indicates the acetate group. A peak observed at 566cm-1 indicates the presence of calcium carbonate and vat rite compound [9]. A peak observed at 566 indicates the phosphate group.
Figure 1: Calcium precipitating bacteria isolated from Rock environment region showing control and the bacillus bacteria is present inside the conical flask.

Figure 2: Fourier-transform infrared spectrum of Control for calcium precipitating bacteria cultured in Nanotechnology laboratory by using isolate collected from rock environment region.
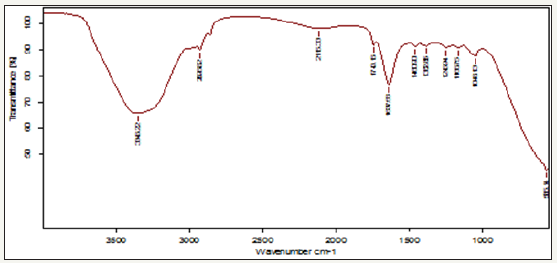
Figure 3: Fourier-transform infrared spectrum for calcium precipitating bacteria cultured in Nanotechnology laboratory by using isolate collected from rock environment region.
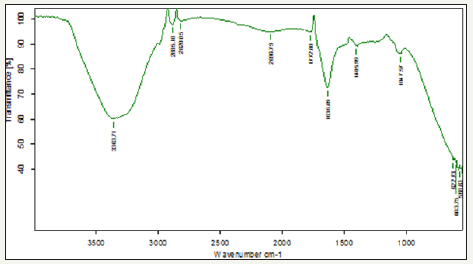
Figure 3 Shows FT-IR spectrum for bacillus bacteria (CPB) cultured at 40 °C. A peak noticed at 3363cm-1indicates OH stretching vibration of carboxylic acids. A peak noticed at 2885 and 2820cm- 1 can be assigned to the C-H stretching vibration of aromatic and alkynes compounds. A peak observed at 2096 cm-1 indicates the N-H stretching vibrations. A peak observed at 1772cm-1 indicates stretching vibration of calcium ion binding with COO groups of carbonyl band. A peak observed at 1636cm-1 indicates the acetate group. A peak observed at 1406 and 1047cm-1 indicates carboxylic dimer stretching vibration of metallic carbonates. A peak observed at 622 and 603cm-1 indicates the presence of calcium carbonate and vaterite. A peak observed at 568cm-1 indicates the phosphate group. CPB systems there is a high intensity peak were observed at 2885cm-1 due to highly deposition of calcium carbonate.
Figure 4: 3a X-Ray diffraction spectrum of control for calcium precipitating bacteria cultured in Nanotechnology laboratory by using isolate collected from rock environment region.
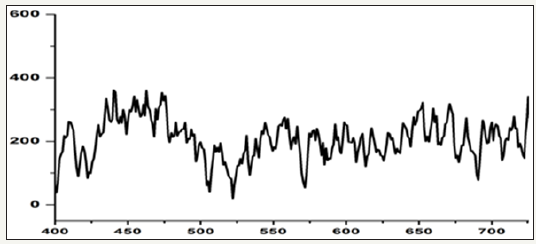
Figure 5: X-Ray diffraction spectrum of calcium precipitating bacteria cultured in Nanotechnology laboratory by using isolate collected from rock environment region.
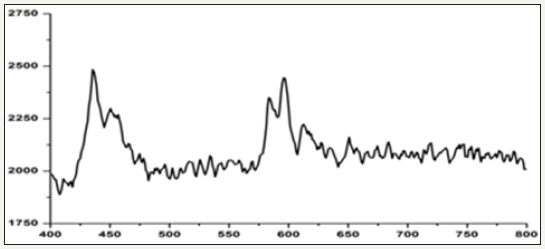
The FT-IR study reveals that the organic functional groups of carboxylic, secondary amines, hydroxyl and phosphate groups are corresponding to the formation of calcium precipitate on the bacterial biofilm. The biogenic functional groups can act as nucleating site for calcium mineralization and scale formation. There are no changes in biogenic functional group during formation of calcium crystal at 37 °C and 40 °C temperatures. The change is seen only in the peak intensity of calcium carbonate crystals (2885cm-1). The formation of calcium carbonate crystals depend upon the biological activities. (Figure 4,5) presents the details of XRD data corresponding to the phases present in the samples collected from rock samples. The following diffraction peaks at 32, 38, 25, 16 and 55 corresponding to άCa2 (P2O7 ), Ca2P6O7 were noticed. The crystal phase of Ca2 (P2O7) peaks was high intensity when compared to control system (without bacteria). The intensity of Ca2P2O7 crystal phase peak depends upon the biological activities and formation of calcium crystals.
Microbial associations with natural carbonate deposits have been described for seawater, saline lakes and soils. The occurrences of human kidney stone development as well as the deposits on the Martian meteorite and the phenomenon of nano forms were hypothesized to be in association with microbial calcification. In this study, the occurrence of crystal formation in rock environments has been reported. The identified isolates showed positive for urease activity. The result supports the observation made by previous investigators, who noticed urease positive in CPB. Hammes & Verstraete [10] also noticed that microbial carbonate precipitation by Synechococcus spp, Nannochloris atomus, Bacillus spp, Pseudomonas spp, Vibrio spp, Flavobacterium spp, and Acinetobacter spp, Photosynthetic-induced calcification is regarded as the most common form of microbial calcium precipitate (MCP), and is associated with algae or cyanobacteria in primarily aqueous environments such as marine and/or freshwater [11].
The presence of CaCO3 and calcium phosphate in XRD peaks indicate the role of bacteria on calcium precipitation. The presence of metallic carbonate and phosphates noticed by FT-IR which indicates the role of bacteria on calcium accumulation. Bouquet et al. [4] showed that most soil bacteria are able to precipitate crystals of calcium carbonate when tested in a medium containing calcium acetate and that calcite production by bacteria is just a function of the media composition. FT-IR reveals the presence of carboxylic acid of ASP and GLU represent binding sites for Ca2+ ions L-Asp resulting in the formation of organic calcium formation.
Conclusion
On the basis of the result, it is concluded that the calcium precipitating bacteria were grown at 40 ᵒC. The temperature is one of the causative factors for crystal formation in B4 medium. This type of bacteria deposit calcium for crystal formation.
References
- Braissant O, verrecchia EP, Aragno M, (2002) Is the contribution of bacteria to terrestrial carbon budget grately underestimated? Naturwissenchaften 89(8): 366-370.
- Knorre H, Krumbein W (2000) Bacterial calcification. In: Riding RR, Awramik SM (Eds.), Microbial sediment, pp. 25-31.
- Morita RY (1980) Calcite precipitation by marine bacteria. Geomicrobiology Journal 2(1): 63-82.
- Boquet E, Boronat A, Ramos CA (1973) Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 246: 527-529.
- Kramer G, Klingler HC, Steiner GE (2000) Role of bacteria in the development of kidney stones. Curr Opin Urol 10(1): 35-38.
- McKay DS, Gibson EK , Thomas Keprta KL, Vali H, Romanek CS, et al. (1996) Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH84001. Science 273(5277): 924-930.
- Fujita Y, Ferris EG, Lawson RD, Colwell FS, Smith RW (2000) Calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiology Journal 17: 305-318.
- Vali H, Mckee MD, Ciftcioglu N, Sears K, Plows F, et al. (2001) Nanoforms: a new type of protein-associated mineralization. Geochim. Cosmochim Acta 65(1): 63-74.
- Wang L, Sondi I, Matijevic E (1999) J. Colloid Interface Sci, Netherlands, p. 218: 545.
- Hammes F, Verstraete W (2002) Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol 1(1): 3-7.
- McConnaughey TA, Whelan FF (1997) Calcification generates protons for nutrient a bicarbonate uptake. Earth Sci Rev 42(1-2): 95-117.
© 2018 TNVKV Prasad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






